Summary
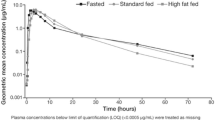
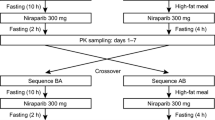
Background Pazopanib is approved for metastatic renal cell carcinoma (RCC). We assessed the safety and efficacy of pazopanib with a low fat meal (LFM): <400 cal and < 20% fat or 10 g per meal. Methods A single arm study of pazopanib with a LFM in 16 adult patients with metastatic RCC with a clear cell component, RECIST 1.1 measurable disease, ECOG PS ≤ 2, and ≤ 3 prior therapies. Pazopanib at 400 mg daily given with LFM for 12 weeks. Incremental dose increases up to 800 mg, or irreversible decreases to 200 mg, allowed every 2 weeks. Primary study endpoint was safety; adverse events (AE) measured per CTCAE version 4.0. Secondary endpoints of RECIST 1.1 response with assessment as 12 weeks; pharmacokinetic (PK) analysis at nine time points, and CYP3A4 polymorphism evaluation. Results Pazopanib with a LFM was well tolerated; 13 of 16 subjects completed all 12 weeks. Three patients withdrew due to adverse events (AEs), with five occurrences of grade 3 AEs. Conclusions Pazopanib with a LFM has acceptable safety and comparable efficacy to fasting administration. Total median pazopanib dose per subject for the study duration was 63.5% of maximum possible conventional dose. A larger study is warranted. Clinical Trial Registration Number: NCT02729194.





Similar content being viewed by others
References
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, de Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–731
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarbá JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6):1061–1068
Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, Malburg L, Arumugham T, Dar MM, Suttle AB, Gainer SD, LoRusso P (2010) A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther 88(6):818–823
Stover JT, Moore RA, Davis K, Harrison MR, Armstrong AJ (2015) Reversal of PSA progression on abiraterone acetate through the administration with food in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 18(2):161–166
Szmulewitz RZ, Peer CJ, Ibraheem A, Martinez E, Kozloff MF, Carthon B, Harvey RD, Fishkin P, Yong WP, Chiong E, Nabhan C, Karrison T, Figg WD, Stadler WM, Ratain MJ (2018) Prospective international randomized phase II study of low-dose Abiraterone with food versus standard dose Abiraterone in castration-resistant prostate Cancer. J Clin Oncol 36(14):1389–1395
Xu CF, Xue Z, Bing N, King KS, McCann LA, de Souza PL et al (2012) Concomitant use of pazopanib and simvastatin increases the risk of transaminase elevations in patients with cancer. Ann Oncol 23(9):2470–2471
Sparidans RW, Ahmed TT, Muilwijk EW, Welzen ME, Schellens JH, Beijnen JH (2012) Liquid chromatography-tandem mass spectrometric assay for therapeutic drug monitoring of the tyrosine kinase inhibitor pazopanib in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 905:137–140
Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M (2007) Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 6(7):2012–2021
Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, Hodge JP, Merkle EM, Pandite L (2009) Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 15(12):4220–4227
Thorn CF, Sharma MR, Altman RB, Klein TE (2017) PharmGKB summary: pazopanib pathway, pharmacokinetics. Pharmacogenet Genomics 27(8):307–312
Verheijen RB, Bins S, Mathijssen RH, Lolkema MP, van Doorn L, Schellens JH et al (2016) Individualized Pazopanib dosing: a prospective feasibility study in Cancer patients. Clin Cancer Res 22(23):5738–5746
Lankheet NAG, Desar IME, Mulder SF, Burger DM, Kweekel DM, van Herpen CML, van der Graaf WTA, van Erp NP (2017) Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol 83(10):2195–2204
Acknowledgements
We gratefully acknowledge the participation of our patients and their families as well the efforts of the University of Michigan Rogel Cancer Center Dieticians Melissa Shannon-Hagan and Nancy Burke, who counseled patients regarding low-fat meals for this study.
Author information
Authors and Affiliations
Contributions
M.A.R. and A.S.A. analyzed and interpreted the data and wrote the manuscript. A.S.A, M.M.S. and B.G. R. designed the study and enrolled patients. S.D.N. conducted statistical analysis. R.D. and L.R. collected patient information. J. A. F. and B.W. conducted pharmacokinetic analysis. S.K. provided clinical pharmacy support. C.G. and J.R. conducted pharmacogenomic analysis. D.K. provided nutrition information and counseled patients.
Corresponding author
Ethics declarations
Ethical approval
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no potential conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Reimers, M.A., Shango, M.M., Daignault-Newton, S. et al. Pazopanib with low fat meal (PALM) in advanced renal cell carcinoma. Invest New Drugs 37, 323–330 (2019). https://doi.org/10.1007/s10637-018-0692-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0692-8