Summary
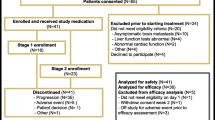
Up-regulation of the Hedgehog (Hh) pathway is implicated in the genesis of a wide range of tumors including triple negative breast cancer (TNBC). Sonidegib is a potent and selective oral inhibitor of Smo, a key component of the Hh signaling pathway. We designed a phase I clinical study to explore the combination of sonidegib plus docetaxel (fixed dose at 75 mg/m2) in advanced TNBC patients. The primary objective was to ascertain the combination’s maximum tolerated dose and the recommended phase II dose (RP2D), based on dose limiting toxicities (DLTs) in the first 2 cycles. A standard “3 + 3” design was followed including three dose levels (DL) of sonidegib: 400 mg (DL1), 600 mg (DL2), and 800 mg (DL3). Twelve patients were included. Sonidegib 800 mg orally q.d. plus docetaxel 75 mg/m2 given intravenously on day 1 of 21-day cycles was established as the RP2D. No DLTs were observed at any DL. The median number of administered cycles at DL3 was 8 (range: 6 to 9). Grade 3 adverse events (AEs) at DL3 were neutropenia (66.7%), CPK increase (33.3%), leukopenia (33.3%), and paresthesia (33.3%), grade 4 AEs were not reported at this DL. At the RP2D, the combination showed antitumor activity in three out of 10 patients with measurable disease. Median time to progression for the overall study was 42.5 days (95% Confidence Interval: 29–155), and 188 days at DL3. No drug-to-drug interactions between sonidegib and docetaxel were found in the PK assessment. Trial Registration: EudraCT study number: 2013–001750-96. Study GEICAM/2012–12. TRIAL REGISTRATION: EudraCT study number: 2013-001750-96. Study GEICAM/2012-12. ClinicalTrials.gov: NCT02027376



Similar content being viewed by others
References
Hon JD, Singh B, Sahin A, Du G, Wang J, Wang VY, Deng FM, Zhang DY, Monaco ME, Lee P (2016) Breast cancer molecular subtypes: from TNBC to QNBC. Am J Cancer Res 6(9):1864–1872
Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA (2015) Subtyping of triple-negative breast cancer: implications for therapy. Cancer 121(1):8–16
Ghersi D, Wilcken N, Simes RJ (2005) A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer 93(3):293–301
Ojima I, Lichtenthal B, Lee S, Wang C, Wang X (2016) Taxane anticancer agents: a patent perspective. Expert Opin Ther Pat 26(1):1–20
Silapunt S, Chen L, Migden MR (2016) Hedgehog pathway inhibition in advanced basal cell carcinoma: latest evidence and clinical usefulness. Ther Adv Med Oncol 8(5):375–382
Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M (2004) Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res 64(17):6071–6074
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66(12):6063–6071
Fiaschi M, Rozell B, Bergstrom A, Toftgard R (2009) Development of mammary tumors by conditional expression of GLI1. Cancer Res 69(11):4810–4817
Habib JG, O’Shaughnessy JA (2016) The hedgehog pathway in triple-negative breast cancer. Cancer Med 5(10):2989–3006
Doan HQ, Silapunt S, Migden MR (2016) Sonidegib, a novel smoothened inhibitor for the treatment of advanced basal cell carcinoma. Onco Targets Ther 9:5671–5678
Chun HW, Hong R (2016) Significance of the hedgehog pathway-associated proteins Gli-1 and Gli-2 and the epithelial-mesenchymal transition-associated proteins Twist and E-cadherin in hepatocellular carcinoma. Oncol Lett 12(3):1753–1762
FDA. (2015). FDA Sonidegib approval. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205266s000lbl.pdf. Accessed 22 March 2007
Steg AD, Katre AA, Bevis KS, Ziebarth A, Dobbin ZC, Shah MM, Alvarez RD, Landen CN (2012) Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol Cancer Ther 11(7):1587–1597
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical, O., & College of American, P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Department of Health and Human Services. National Institutes of Health. National Cancer Institute. (May 28, 2009 (v4.03: June 14, 2010)). Common Terminology Criteria for Adverse Events (CTCAE)
G., R. (2015). LC-MS/MS Standard Operating Procedure for the quantitative determination of LDE225 in human plasma. SGS Cephac Europe Analytical Method SOP No. 1970 Version d
Goel V, Hurh E, Stein A, Nedelman J, Zhou J, Chiparus O, Huang PH, Gogov S, Sellami D (2016) Population pharmacokinetics of sonidegib (LDE225), an oral inhibitor of hedgehog pathway signaling, in healthy subjects and in patients with advanced solid tumors. Cancer Chemother Pharmacol 77(4):745–755
Ocana A, Pandiella A (2017) Targeting oncogenic vulnerabilities in triple negative breast cancer: biological bases and ongoing clinical studies. Oncotarget 8(13):22218–22234
Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, Montastruc M, Eche A, Vigarios E, Dalenc F, Lacouture ME (2016) Dermatological adverse events with taxane chemotherapy. Eur J Dermatol 26(5):427–443
Nakatsukasa K, Koyama H, Oouchi Y, Imanishi S, Mizuta N, Sakaguchi K, Fujita Y, Fujiwara I, Kotani T, Matsuda T, Fukuda K, Morita M, Kawakami S, Kadotani Y, Konishi E, Yanagisawa A, Taguchi T (2017) Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer 24(1):63–68
Mustacchi G, De Laurentiis M (2015) The role of taxanes in triple-negative breast cancer: literature review. Drug Des Devel Ther 9:4303–4318
Khatra H, Bose C, Sinha S (2017) Discovery of Hedgehog Antagonists for Cancer Therapy. Curr Med Chem 24(19):2033–2058
Ramelyte E, Amann VC, Dummer R (2016) Sonidegib for the treatment of advanced basal cell carcinoma. Expert Opin Pharmacother 17(14):1963–1968
Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, Sarantopoulos J, Mahalingam D, Shou Y, Moles MA, Yang L, Granvil C, Hurh E, Rose KL, Amakye DD, Dummer R, Mita AC (2014) A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res 20(7):1900–1909
Stathis A, Hess D, von Moos R, Homicsko K, Griguolo G, Joerger M, Mark M, Ackermann CJ, Allegrini S, Catapano CV, Xyrafas A, Enoiu M, Berardi S, Gargiulo P, Sessa C, Swiss Group for Clinical Cancer, R (2017) Phase I trial of the oral smoothened inhibitor sonidegib in combination with paclitaxel in patients with advanced solid tumors. Investig New Drugs 35(6):766–772
Acknowledgments
Authors thank all participating patients and their families, as well as the whole network of investigators, research nurses, study coordinators, central labs and GEICAM staff, with special mention to Mª José Escudero, Ruth Caro, Ana Blach and Cristina del Monte from the GEICAM staff.
Funding
Novartis Farmacéutica, S.A. provided financial support and the study drug.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Federico Rojo: AES Program, grant PI15/00934 and grant PT17/0015/0006, ISCIII. Eva Carrasco’s husband is Novartis advisor for onco-hematology products. Rest of the authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ruiz-Borrego, M., Jimenez, B., Antolín, S. et al. A phase Ib study of sonidegib (LDE225), an oral small molecule inhibitor of smoothened or Hedgehog pathway, in combination with docetaxel in triple negative advanced breast cancer patients: GEICAM/2012–12 (EDALINE) study. Invest New Drugs 37, 98–108 (2019). https://doi.org/10.1007/s10637-018-0614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0614-9