Summary
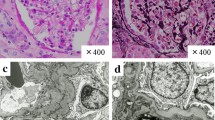
We here report a case of nivolumab-induced acute granulomatous tubulointerstitial nephritis in a patient with gastric cancer. A 68-year-old woman with recurrent gastric cancer developed acute kidney injury associated with kidney enlargement and urinary leukocytes after 38 cycles of nivolumab treatment. A diagnosis of acute granulomatous tubulointerstitial nephritis was made based on kidney biopsy findings. Immunohistochemistry revealed expression of programmed cell death–ligand 1 (PD-L1) in degenerated epithelial cells of collecting tubules. Among infiltrating immune cells, aggregation of T cells was more extensive than that of B cells, with CD4+ T cells outnumbering CD8+ T cells, consistent with the relative numbers of these cells in the circulation. Treatment with methylprednisolone (1.0 mg/kg daily) led to a rapid improvement in renal function and reduction in the number of circulating CD4+ T cells. Prompt administration of high-dose corticosteroid is thus recommended after diagnosis of this adverse event of nivolumab treatment by kidney biopsy.




Similar content being viewed by others
References
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461–2471
Opdivo (Nivolumab). https://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed 1 Mar 2018
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P (2015) Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced Nonsquamous non-small-cell lung Cancer. N Engl J Med 373:1627–1639
Chau I, Chen LT, Kang YK et al (2018) Nivolumab safety profile in Asian and western patients with chemotherapy-refractory (CTx-R) advanced gastric/gastroesophageal junction (adv G/GEJ) cancer from the ATTRACTION-2 and CheckMate-032 trials. J Clin Oncol 36:2018 (suppl 4S; abstr 90)
Belliere J, Meyer N, Mazieres J, Ollier S, Boulinguez S, Delas A, Ribes D, Faguer S (2016) Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer 115:1457–1461
Escandon J, Peacock S, Trabolsi A, Thomas DB, Layka A, Lutzky J (2017) Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother Cancer 5:3
Izzedine H, Mateus C, Boutros C et al (2017) Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant 32:936–942
Chen Y, Zhang J, Li J, Zou L, Zhao T, Tang Y, Wu Y (2006) Expression of B7-H1 in inflammatory renal tubular epithelial cells. Nephron Exp Nephrol 102:e81–e92
Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE (1990) MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int 37:783–792
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167–3175
Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD, on behalf of Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors (2017) Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 45:160–169
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K (2018) Association of Immune-Related Adverse Events with Nivolumab Efficacy in non-small-cell lung Cancer. JAMA Oncol 4:374–378
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yoshihisa Nakatani declares that he has no conflict of interest. Hisato Kawakami has received honoraria from Ono Pharmaceutical Co. Ltd. Masashi Ichikawa declares that he has no conflict of interest. Sachiyo Yamamoto declares that she has no conflict of interest. Yasuo Otsuka declares that he has no conflict of interest. Akiko Mashiko declares that she has no conflict of interest. Yasutoshi Takashima declares that he has no conflict of interest. Akihiko Ito declares that he has no conflict of interest. Kazuhiko Nakagawa has received honoraria and research funding from Ono Pharmaceutical Co. Ltd. and Bristol Myers Squibb Co. as well as consulting fees from Ono Pharmaceutical Co. Ltd. Shuji Arima declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nakatani, Y., Kawakami, H., Ichikawa, M. et al. Nivolumab-induced acute granulomatous tubulointerstitial nephritis in a patient with gastric cancer. Invest New Drugs 36, 726–731 (2018). https://doi.org/10.1007/s10637-018-0596-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0596-7