Summary
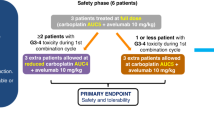
Background MLN0128 is a first-in-class, dual mTOR inhibitor with potential to outperform standard rapalogs through inhibition of TORC1 and TORC2. This phase II study was designed to assess antitumor activity of MLN0128 in metastatic castration-resistant prostate cancer (mCRPC). Methods Eligible patients had mCRPC previously treated with abiraterone acetate and/or enzalutamide. Five patients started MLN0128 at 5 mg once daily, subsequently dose reduced to 4 mg because of toxicity. Four subsequent patients started MLN0128 at 4 mg daily. Primary endpoint was progression-free survival at 6 months. Results Nine patients were enrolled and median time on treatment was 11 weeks (range: 3–30). Best response was stable disease. All patients had a rise in PSA on treatment, with a median 159% increase from baseline (range: 12–620%). Median baseline circulating tumor cell count was 1 cell/mL (range: 0–40); none had a decrease in cell count posttreatment. Grade ≤ 2 adverse events included fatigue, anorexia, and rash. The most common serious adverse events were grade 3 dyspnea and maculopapular rash. Eight patients discontinued treatment early because of radiographic progression (n = 1), grade 3 toxicity (n = 5), or investigator discretion (n = 2). Four patients had immediate PSA decline following drug discontinuation, suggesting MLN0128 could cause compensatory increase of androgen receptor (AR) activity. Correlative studies of pretreatment and posttreatment biopsy specimens revealed limited inhibition of AKT phosphorylation, 4EBP1 phosphorylation, and eIF4E activity. Conclusions Clinical efficacy of MLN0128 in mCRPC was limited likely due to dose reductions secondary to toxicity, PSA kinetics suggesting AR activation resulting from mTOR inhibition, and poor inhibition of mTOR signaling targets.





Similar content being viewed by others
References
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell. https://doi.org/10.1016/j.ccr.2010.05.026
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell. 169(2):361–371. https://doi.org/10.1016/j.cell.2017.03.035
Hsieh AC, Truitt ML, Ruggero D (2011) Oncogenic AKTivation of translation as a therapeutic target. Br J Cancer. https://doi.org/10.1038/bjc.2011.241
Edlind MP, Hsieh AC (2014) PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. https://doi.org/10.4103/1008-682X.122876
Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP (2009) Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. https://doi.org/10.1126/scisignal.2000189
Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. https://doi.org/10.1038/nature10912
Chiu MI, Katz H, Berlin V (1994) RAPT1, a mammalian homolog of yeast tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci U S A 91(26):12574–8
Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP (2013) mTOR kinase structure, mechanism and regulation. Nature. https://doi.org/10.1038/nature12122
Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. https://doi.org/10.1038/nm1052
Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, Gautschi O, Borner M, Fechter E, Stenner F, Winterhalder R, Muller B, Schiess R, Wild PJ, Ruschoff JH, Thalmann G, Dietrich PY, Aebersold R, Klingbiel D, Gillessen S, Swiss Group for Clinical Cancer Research (SAKK) (2013) Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol. https://doi.org/10.1016/j.eururo.2013.03.040
Amato RJ, Jac J, Mohammad T, Saxena S (2008) Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. https://doi.org/10.3816/CGC.2008.n.015
Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood DP, Creel PA, Mundy K, Davis SL, Wang T, Albadine R, Schultz L, Partin AW, Jimeno A, Fedor H, Febbo PG, George DJ, Gurganus R, De Marzo AM, Carducci MA (2010) A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-10-0124
Rathkopf DE, Larson SM, Anand A, Morris MJ, Slovin SF, Shaffer DR, Heller G, Carver B, Rosen N, Scher HI (2015) Everolimus combined with gefitinib in patients with metastatic castration-resistant prostate cancer: phase 1/2 results and signaling pathway implications. Cancer. https://doi.org/10.1002/cncr.29578
Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0809136105
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65(16):7052–8
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. https://doi.org/10.1371/journal.pbio.1000038
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. https://doi.org/10.1074/jbc.M900301200
Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D (2010) Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. https://doi.org/10.1016/j.ccr.2010.01.021
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. https://doi.org/10.1200/JCO.2007.12.4487
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. https://doi.org/10.1016/j.ejca.2008.10.026
Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O'Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF (2015) Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. https://doi.org/10.1016/j.jmoldx.2014.12.006
Hsieh AC, Nguyen HG, Wen L, Edlind MP, Carroll PR, Kim W, Ruggero D (2015) Cell type-specific abundance of 4EBP1 primes prostate cancer sensitivity or resistance to PI3K pathway inhibitors. Sci Signal. https://doi.org/10.1126/scisignal.aad5111
Infante JR, Tabernero J, Cervantes A, Jalal S, Burris HA, Macarulla T, Perez-Fidalgo JA, Neuwirth R, Patel C, Gangolli E, Brake R, Sturm J, Westin EH, Gordon M (2013) Abstract C252: a phase 1, dose-escalation study of MLN0128, an investigational oral mammalian target of rapamycin complex 1/2 (mTORC1/2) catalytic inhibitor, in patients (pts) with advanced non-hematologic malignancies. Mol Cancer Ther 12
Werner SL, Graf RP, Landers M, Valenta DT, Schroeder M, Greene SB, Bales N, Dittamore R, Marrinucci D (2015) Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circ Biomark. https://doi.org/10.5772/60725
Wu L, Birle DC, Tannock IF (2005) Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 65(7):2825–31
Burris HA 3rd, Kurkjian CD, Hart L, Pant S, Murphy PB, Jones SF, Neuwirth R, Patel CG, Zohren F, Infante JR (2017) TAK-228 (formerly MLN0128), an investigational dual TORC1/2 inhibitor plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-017-3343-4
Ghobrial IM, Siegel DS, Vij R, Berdeja JG, Richardson PG, Neuwirth R, Patel CG, Zohren F, Wolf JL (2016) TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: a phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenstrom's macroglobulinemia. Am J Hematol. https://doi.org/10.1002/ajh.24300
Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E, Barratt DG, Cosulich S, Klinowska T, Rosen N, Shokat KM (2016) Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. https://doi.org/10.1038/nature17963
Fan Q, Aksoy O, Wong RA, Ilkhanizadeh S, Novotny CJ, Gustafson WC, Truong AY, Cayanan G, Simonds EF, Haas-Kogan D, Phillips JJ, Nicolaides T, Okaniwa M, Shokat KM, Weiss WA (2017) A kinase inhibitor targeted to mTORC1 drives regression in glioblastoma. Cancer Cell 31(3):424–435. https://doi.org/10.1016/j.ccell.2017.01.014
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL (2011) Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. https://doi.org/10.1016/j.ccr.2011.04.008
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, Plaisier S, Garraway IP, Huang J, Graeber TG, Wu H (2011) Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. https://doi.org/10.1016/j.ccr.2011.05.006
Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, Scher HI, Barry ST, Sawyers CL, Chandarlapaty S, Rosen N (2015) Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. https://doi.org/10.1016/j.ccell.2014.11.008
de Bono, JS, De Girogi U, Massard C, Bracarda S, Nava Rodrigues D, Kocak I et al (2016) PTEN loss as a predictive biomarker for the Akt inhibitor ipatasertib combined with abiraterone acetate in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol: Official J Eur Soc Med Oncol/ ESMO suppl_6:7180
Sheridan CM, Grogan TR, Nguyen HG, Galet C, Rettig MB, Hsieh AC, Ruggero D (2015) YB-1 and MTA1 protein levels and not DNA or mRNA alterations predict for prostate cancer recurrence. Oncotarget 6(10):7470–80
Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, Foye A, Lloyd P, Nykter M, Beer TM, Alumkal JJ, Thomas GV, Reiter RE, Rettig MB, Evans CP, Gao AC, Chi KN, Small EJ, Gleave ME (2017) Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx118
Funding
D.E.R. received funding and drug supply from Millenium Pharmaceuticals to support this trial. K.B. is a recipient of an American Society of Clinical Oncology Young Investigator Award and is funded by a National Cancer Institute training grant (T32CA009515) and a Pilot and Feasibility Studies Program grant funded by the Co-operative Center for Excellence in Hematology. B.S.C. is funded by 1R01 CA182503-01A1. T.L.L. was funded in part by a Transformative Impact Award from the CDMRP (W81XWH-12-PCRP-TIA). A.C.H. is a V Foundation Scholar and is funded by a NextGen Grant for Transformative Cancer Research from the American Association for Cancer Research, a Fred Hutchinson Cancer Research Center/University of Washington Cancer Center Support Grant, a National Institutes of Health Career Development Award (1K08CA175154–01), and the Burroughs Wellcome Fund. D.E.R., Y.C., and H.I.S. received support through the NIH/NCI Cancer Center Support Grant P30 CA008748. B.S.C, Y.C, A.C.H., and D.E.R. are recipients of a Movember-Prostate Cancer Foundation Challenge Award. NCI P50CA092629 (B.S.C., Y.C., H.I.S., D.E.R), NCI P30CA008748 (B.S.C., Y.C., H.I.S., D.E.R).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
D.E.R. is a consultant for Janssen (uncompensated) and receives research funding from Astellas, Astra-Zeneca, Celgene, Genentech, Janssen, Medivation, Novartis, Taiho, Tracon. L.G. declares that she has no conflicts of interest. K.B. declares that he has no conflicts of interest. A.T. declares that she has no conflicts of interest. B.S.R. declares that he has no conflicts of interest., Y.C. declares that he has no conflicts of interest. K.P. declares that she has no conflicts of interest. G.S. declares that he has no conflicts of interest. H.I.S. declares that he has no conflicts of interest. T.L.L. declares that she has no conflicts of interest. A.C.H. declares that he has no conflicts of interest.
Research involving human participants and/or animals
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Graham, L., Banda, K., Torres, A. et al. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Invest New Drugs 36, 458–467 (2018). https://doi.org/10.1007/s10637-018-0578-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0578-9