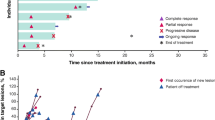
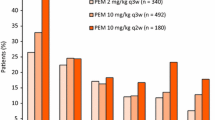
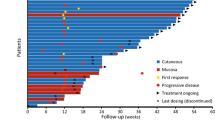
Summary
Objectives Ontuxizumab (MORAB-004) is a first-in-class monoclonal antibody that interferes with endosialin function, which is important in tumor stromal cell function, angiogenesis, and tumor growth. This Phase 2 study evaluated the 24-week progression-free survival (PFS) value, pharmacokinetics, and tolerability of 2 doses of ontuxizumab in patients with metastatic melanoma. Patients and methods Patients with metastatic melanoma and disease progression after receiving at least 1 prior systemic treatment were randomized to receive ontuxizumab (2 or 4 mg/kg) weekly, without dose change, until disease progression. Results Seventy-six patients received at least 1 dose of ontuxizumab (40 received 2 mg/kg, 36 received 4 mg/kg). The primary endpoint, 24-week PFS value, was 11.4% (95% Confidence Interval [CI]: 5.3%–19.9%) for all patients (13.5% for 2 mg/kg and 8.9% for 4 mg/kg). The median PFS for all patients was 8.3 weeks (95% CI: 8.1–12.3 weeks). One patient receiving 4 mg/kg had a partial response, as measured by Response Evaluation Criteria in Solid Tumors v1.1. Twenty-seven of 66 response evaluable patients (40.9%) had stable disease. The median overall survival was 31.0 weeks (95% CI: 28.3–44.0 weeks). The most common adverse events overall were headache (55.3%), fatigue (48.7%), chills (42.1%), and nausea (36.8%), mostly grade 1 or 2. Conclusions Ontuxizumab at both doses was well tolerated. The 24-week PFS value was 11.4% among all ontuxizumab-treated patients. The overall response rate was 3.1% at the 4 mg/kg dose, with clinical benefit achieved in 42.4% of response evaluable patients. Efficacy of single-agent ontuxizumab at these doses in melanoma was low.



Similar content being viewed by others
References
Niezgoda A, Niezgoda P, Czakowski R (2015) Novel approaches to treatment of advanced melanoma: A review of targeted therapy and immunotherapy. Biomed Res Int 2015:851387
Karimkhani C, Gonzalez R, Dellavalle RP (2014) A review of novel therapies for melanoma. Am J Clin Dermatol 2014(4):323–327
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3, randomised controlled trial. Lancet 380(9839):358–365
Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367(2):107–114
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M et al (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371(20):1867–1876
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369(2):134–144
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Andtbacka RH, Kaufman HL, Collichio F, Anatruda T, Senzer N, Chesney J et al (2015) Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788
Flaherty KT, Infante JR, Daud A, Kefford RF, Sosman J, Hamid O et al (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133
St. Croix B, Rago C, Velculescu V, Traversos G, Romans KE, Montgomery E et al (2000) Genes expressed in human tumor endothelium. Science 289:1197–1202
Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C et al (2006) Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci U S A 103:3351–3356
Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D et al (2008) Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol 172:486–494
Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ (1992) Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A 89:10832–10836
Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J et al (2008) Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res 14:7223–7236
Brady J, Neal J, Sadakar N, Gasque P (2004) Human endosialin (tumor endothelial marker 1) is abundantly expressed in highly malignant and invasive brain tumors. J Neuropathol Exp Neurol 63:1274–1283
Huber MA, Kraut N, Schweifer N, Dolznig H, Peter RU, Schubert RD et al (2006) Expression of stromal cell markers in distinct compartments of human skin cancers. J Cutan Pathol 33:145–155
Kiyohara E, Donovan N, Takeshima L, Huang S, Wilmott JS, Scolyer RA et al (2015) Endosialin Expression in Metastatic Melanoma Tumor Microenvironment Vasculature: Potential Therapeutic Implications. Cancer Microenviron 8(2):111–118
Gnjatic S, Wheeler C, Ebner M, Ritter E, Murray A, Altorki NK et al (2009) Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods 341:50–58
Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB et al (2007) An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 10:35–45
Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L et al (2010) Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther 9:908–915
Bagley RG, Rouleau C, St. Martin T, Boutin P, Weber W, Ruzek M et al (2008) Human endosialin: precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther 7:2536–2546
Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E et al (2007) Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A 104:17965–17970
Diaz LA, Coughlin CM, Weil SC, Fishel J, Gounder MM, Lawrence S et al (2015) A first-in-human phase 1 study of MORAB-004, a monoclonal antibody to endosialin in patients with advanced solid tumors. Clin Cancer Res 21(6):1281–1288
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Kalbfleisch JD, Prentice RL (2001) The statistical analysis of failure time data. John Wiley & Sons, Inc, New York
Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38:29–41
O’Shannessy DJ, Smith MF, Somers EB, Jackson SM, Albone E, Tomkowicz B et al (2016) Novel Antibody Probes for the Characterization of Endosialin/TEM-1. Oncotarget 7(43):69420–69435
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C et al (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol 16:908–918
Krauthauser C, Grasso L, Nicolaides N, Lin JM (2012) Neutralizing endosialin antibody, MORAb-004, enhances efficacy of TAXOTERE in a metastatic melanoma model [abstract]. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; Cancer Res 72(8 Suppl): Abstract No. 4410
Deichmann M, Benner A, Bock M, Jackel A, Uhl K, Waldmann V et al (1999) S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol 17:1891–1896
Rybinski K, Imtiyaz HC, Mittica B, Drozdowski B, Fulmer J, Furuuchi K et al (2015) Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature. Oncotarget 6(28):25429–25440
Acknowledgements
Medical writing assistance was provided by M.L. Skoglund, PhD; Healthcare Consulting.
Funding
This study was funded by Morphotek, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sandra D’Angelo has no conflicts of interest to report.
Omid A. Hamid is consulting for Amgen, Novartis, Roche, BMS and Merck.
Ahmad Tarhini has received a grant from Morphotek, BMS and Merck, is consulting for BMS and Merck.
Dirk Schadendorf has board membership and consulting GSK, Roche, BMS, Amgen, Novartis, Merck, Pfizer, Astra Zeneca. He also has Grant support from BMS and MSD/Merck.
Bartosz Chmielowski is consulting Genentech, Amgen, BMS, Astella, Merck, Eisai, Immunocore, Lilly; he received payment for lectures and travel from Genetech, Janseen and Amgen.
Frances A. Collichio has received grant support from Morphotek, Amgen, Novartis, Vascular Biogenics Ltd., GSK, Amgen, Mayo Phase II and is consulting for Amgen.
Anna C. Pavlick is consulting for BMS and Merck and development of educational presentations from Novartis.
Karl D. Lewis has received grant support from Morphotek.
Susan C. Weil is employed by Morphotek.
John Heyburn was employed by Morphotek.
Charles Schweizer was employed by Morphotek.
Daniel J. O’Shannessy was employed by Morphotek.
Richard Carvajal is consulting for AstraZeneca, BMS, Iconic Therapeutics, Janssen, Merck, Novartis, Roche/Genentech, Thomson Reuters and is board member of Aura Biosciences, Chimeron, Rgenix.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
D’Angelo, S.P., Hamid, O.A., Tarhini, A. et al. A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic melanoma. Invest New Drugs 36, 103–113 (2018). https://doi.org/10.1007/s10637-017-0530-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0530-4