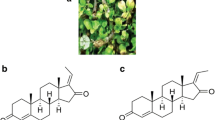
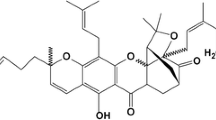
Summary
Cancer is a multifactorial disease, causing behavioral and metabolic alterations, leading to uncontrolled cell proliferation with collateral weakening of immune system. Crucial balance between cell proliferation and cell death determines the fate of a cell, which might progress towards survival or apoptosis. Apoptosis is a complex, programmed, and highly regulated process causing dramatic morphological and biochemical perturbations in the cellular machinery. Ganoderma lucidum is a basidiomycetes, polypore mushroom known for its pharmacological properties in cancer. The major bioconstituents in G. lucidum are terpenoids, polysaccharides, and proteins that target cancer-signaling factors like plasma membrane receptors proteins and adapter molecules. Of all constituents, the major terpenoid, i.e. Ganoderic acid is reported to interact with membrane receptors mainly, receptor tyrosine kinase (RTKs). Ganoderic acid interacts and modulates the signaling network in IR, IGFR-1, IGFR-2, VEGFR-1, VEFGR-2, and EGFR in cancer signaling pathways. It primarily targets NF-κB, RAS-MAPK, PI3K/Akt/mTOR, and cell cycle resulting in apoptosis. This review highlights the role of ganoderic acid in apoptosis and modulations of various signaling proteins in cancer.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Negi A, Gill B, Anand S (2014) Tilling: versatile reverse genetic tool. PharmaTutor 2(1):26–32
Arap W, Pasqualini R, Ruoslahti E (1998) Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279(5349):377–380
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years⊥. J Nat Prod 70(3):461–477
Gill BS, Navgeet, Kumar S (2017) Ganoderma lucidum targeting lung cancer signaling: a review. Tumor Biol 39(6):1010428317707437
Kerr J (1965) A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol 90(2):419–435
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239
Cotter TG (2009) Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 9(7):501–507
Gill BS, Alex JM, Navgeet, Kumar S (2016) Missing link between microRNA and prostate cancer. Tumor Biol 37(5):5683–5704
Schulze-Osthoff K, Walczak H, Dröge W, Krammer PH (1994) Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol 127(1):15–20
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135(6):1441–1455
Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF (2005) Actin-myosin–based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168(2):245–255
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin-and myosin-dependent mechanism. Curr Biol 11(23):1847–1857
Anand SS, Gill BS (2015) Breakthroughs in epigenetics. PharmaTutor 3(7):16–24
Napirei M, Karsunky H, Zevnik B, Stephan H, Möröy T (2000) Features of systemic lupus erythematosus in Dnase1-deficient mice. Nature Genet 25(2):177–181
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391(6662):43–50
Ramirez IB-R, Lowe M (2009) Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol 20(7):770–779
Ricci J-E, Muñoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR (2004) Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117(6):773–786
Lüthi A, Martin S (2007) The CASBAH: a searchable database of caspase substrates. Cell Death Differ 14(4):641–650
Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA (1997) Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol 159(11):5391–5399
Moss DK, Betin VM, Malesinski SD, Lane JD (2006) A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci 119(11):2362–2374
Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392(6671):86–89
Gill BS, Kumar S (2016) Triterpenes in cancer: significance and their influence. Mol Biol Rep 43(9):881–896
Gill BS, Kumar S (2016) Ganoderic acid a targeting β-catenin in Wnt signaling pathway: in silico and in vitro study. Interdiscip Sci Comput Life Sci 1–11. https://doi.org/10.1007/s12539-016-0182-7
Tavakoli J, Miar S, Zadehzare MM, Akbari H (2012) Evaluation of effectiveness of herbal medication in cancer care: a review study. Iran J Cancer Prev 5(3):144–156
Moncalvo J (2000) Systematics of Ganoderma 2. In: Flood J, Bridge PD, Holderness M (eds) Ganoderma diseases of perennial crops. CBAI Publishing, U.S.A., p 23
Bhosle S, Ranadive K, Bapat G, Garad S, Deshpande G, Vaidya J (2010) Taxonomy and diversity of Ganoderma from the western parts of Maharashtra (India). Mycosphere 1(3):249–262
Nasir N (2005) Diseases caused by Ganoderma spp. on perennial crops in Pakistan. Mycopathologia 159(1):119–121
Gill BS, Sharma P, Kumar S (2016) Chemical composition and Antiproliferative, antioxidant, and Proapoptotic effects of fruiting body extracts of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), from India. Int J Med Mushrooms 18(7):599–607
Shiao MS (2003) Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec 3(3):172–180
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343(6257):425
Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME (1999) Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med 221(4):281–293
Chen AW, Miles PG (1996) Biomedical research and the application of mushroom nutriceuticals from Ganoderma lucidum. Mushroom Biology and Mushroom Products Pennsylvania State University, Pennsylvania, 161–175
Liu GT (1999) Recent advances in research of pharmacology and clinical applications of Ganoderma P. Karst. species (Aphyllophoromycetideae) in China. Int J Med Mushrooms 1(1):63–68
Chen R, Yu D (1990) Development of triterpenes from Ganoderma lucidum. Acta Pharm Sin 25:940–953
Gill BS, Sharma P, Kumar R, Kumar S (2016) Misconstrued versatility of Ganoderma lucidum: a key player in multi-targeted cellular signaling. Tumor Biol 37(3):2789–2804
Feng L, Yuan L, Du M, Chen Y, Zhang M-H, J-F G, He J-J, Wang Y, Cao W (2013) Anti-lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.) Karst. Molecules 18(8):9966–9981
Chen Y, Lv J, Li K, Xu J, Li M, Zhang W, Pang X (2016) Sporoderm-broken spores of Ganoderma lucidum inhibit the growth of lung cancer: involvement of the Akt/mTOR signaling pathway. Nutr Cancer 68(7):1151–1160
Lin S-B, Li C-H, Lee S-S, Kan L-S (2003) Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci 72(21):2381–2390
Kim TH, Kim JS, Kim ZH, Huang RB, Wang RS (2014) Khz (fusion of Ganoderma lucidum and Polyporus umbellatus mycelia) induces apoptosis in A549 human lung cancer cells by generating reactive oxygen species and decreasing the mitochondrial membrane potential. Food Sci Biotechnol 23(3):859–864
Gill BS, Kumar S (2017) Ganoderic acid modulating TNF and its receptors: in silico and in vitro study. Med Chem Res 26(6):1336–1348
Liu R-M, Zhong J-J (2011) Ganoderic acid mf and S induce mitochondria mediated apoptosis in human cervical carcinoma HeLa cells. Phytomedicine 18(5):349–355
Tang W, Liu J-W, Zhao W-M, Wei D-Z, Zhong J-J (2006) Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci 80(3):205–211
Chen N-H, Liu J-W, Zhong J-J (2010) Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol Rep 62(1):150
J-W X, Zhao W, Zhong J-J (2010) Biotechnological production and application of ganoderic acids. Appl Microbiol Biotechnol 87(2):457–466
Zhou L, Shi P, Chen N-H, Zhong J-J (2011) Ganoderic acid Me induces apoptosis through mitochondria dysfunctions in human colon carcinoma cells. Process Biochem 46(1):219–225
Chen N-H, Liu J-W, Zhong J-J (2008) Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J Pharmacol Sci 108(2):212–216
Jiang J, Grieb B, Thyagarajan A, Sliva D (2008) Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-κB signaling. Int J Mol Med 21(5):577–584
Gill BS, Kumar S (2016) Ganoderic acid targeting multiple receptors in cancer: in silico and in vitro study. Tumor Biol 37(10):14271–14290
Gill BS, Kumar S (2015) Differential algorithms-assisted molecular modeling-based identification of mechanistic binding of ganoderic acids. Med Chem Res 24(9):3483–3493
Gill BS, Kumar S (2016) Evaluating anti-oxidant potential of ganoderic acid a in STAT 3 pathway in prostate cancer. Mol Biol Rep 43(12):1411–1422
G-S W, J-J L, Guo J-J, Li Y-B, Tan W, Dang Y-Y, Zhong Z-F, Z-T X, Chen X-P, Wang Y-T (2012) Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 83(2):408–414
Chen N-H, Zhong J-J (2009) Ganoderic acid Me induces G 1 arrest in wild-type p53 human tumor cells while G 1/S transition arrest in p53-null cells. Process Biochem 44(8):928–933
Li C-H, Chen P-Y, Chang U-M, Kan L-S, Fang W-H, Tsai K-S, Lin S-B (2005) Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci 77(3):252–265
Yue Q-X, Cao Z-W, Guan S-H, Liu X-H, Tao L, W-Y W, Li Y-X, Yang P-Y, Liu X, Guo D-A (2008) Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol Cell Proteomics 7(5):949–961
Marshall C (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80(2):179–185
Joshi G, Singh PK, Negi A, Rana A, Singh S, Kumar R (2015) Growth factors mediated cell signalling in prostate cancer progression: implications in discovery of anti-prostate cancer agents. Chem Biol Interact 240:120–133
Harhaji Trajković LM, Mijatović SA, Maksimović-Ivanić DD, Stojanović ID, Momčilović MB, Tufegdžić SJ, Maksimović VM, Marjanovi ŽS, Stošić-Grujičić SD (2009) Anticancer properties of Ganoderma lucidum methanol extracts in vitro and in vivo. Nutr Cancer 61(5):696–707
Liu R-M, Li Y-B, Zhong J-J (2012) Cytotoxic and pro-apoptotic effects of novel ganoderic acid derivatives on human cervical cancer cells in vitro. Eur J Pharmacol 681(1):23–33
Gill BS, Kumar S, Navgeet (2017) Ganoderic acid targeting nuclear factor erythroid 2–related factor 2 in lung cancer. Tumor Biol 39 (3):1010428317695530
Zhao H, Zhang Q, Zhao L, Huang X, Wang J, Kang X (2011) Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Alternat Med 2012. https://doi.org/10.1155/2012/809614
Soo TS (1996) Effective dosage of the extract of Ganoderma lucidum in the treatment of various ailments. Mushroom biology and mushroom products. In: Royse DJ (ed). The Pennsylvania State University, University Park pp 177–185
Wanmuang H, Leopairut J, Kositchaiwat C, Wananukul W, Bunyaratvej S (2007) Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J Med Assoc Thail 90(1):179
Kawagishi H, Fukuhara F, Sazuka M, Kawashima A, Mitsubori T, Tomita T (1993) 5′-Deoxy-5′-methylsulphinyladenosine, a platelet aggregation inhibitor from Ganoderma lucidum. Phytochemistry 32(2):239–241
Acknowledgements
The authors are thankful to Central University of Punjab, India, for providing the necessary facilities to carry out this work. RM and VK acknowledge ICMR and UGC, respectively, for providing the financial assistance as JRF.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethical approval
This article does not required any ethical approval.
Rights and permissions
About this article
Cite this article
Gill, B.S., Navgeet, Mehra, R. et al. Ganoderic acid, lanostanoid triterpene: a key player in apoptosis. Invest New Drugs 36, 136–143 (2018). https://doi.org/10.1007/s10637-017-0526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0526-0