Summary
Background Although safety and prognostic factors for overall survival (OS) have been extensively studied in Phase I clinical trials on patients with solid tumours, data on lymphoma trials are scarce. Here, we investigated safety, outcomes and prognostic factors in relapsed or refractory lymphoma patients included in a series of Phase I trials. Method and patients All consecutive adult patients with recurrent/refractory lymphoma enrolled in 26 Phase I trials at a single cancer centre in France between January 2008 and June 2016 were retrospectively assessed. Results 133 patients (males: 65%) were included in the analysis. The median (range) age was 65 (23–86). Aggressive non-Hodgkin, indolent non-Hodgkin and Hodgkin types accounted for 64%, 25% and 11% of the patients, respectively. The patients had received a median (range) of 3 (1–13) lines of treatment prior to trial entry. The median [95% confidence interval] progression-free survival and OS times were 3.0 [1.8–3.6] and 17.8 [12.7–30.4] months, respectively. High-grade toxicity (grade 3 or higher, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events classification) was experienced by 56 of the 133 patients (42%) and was related to the investigational drug in 44 of these cases (79%). No toxicity-related deaths occurred. Dose-limiting toxicity (DLT) was encountered in 11 (9%) of the 116 evaluable patients. High-grade toxicity occurred during the DLT period for 34 of the 56 patients (61%) and after the DLT period in the remaining 22 (39%). The main prognostic factors for poor OS were the histological type (i.e. tumour aggressiveness), an elevated serum LDH level, and a low serum albumin level. Early withdrawal from a trial was correlated with the performance status score, the histological type and the serum LDH level. The overall objective response and disease control rates were 24% and 57%, respectively. Conclusion Performance status, LDH, albumin and histological type (tumour aggressiveness) appear to be the most relevant prognostic factors for enrolling Phase I participants with relapsed or refractory lymphoma. 39% of the patients experienced a first high-grade toxic event after the dose-limiting toxicity period, suggesting that the conventional concept of dose-limiting toxicity (designed for chemotherapy) should be redefined in the era of modern cancer therapies.





Similar content being viewed by others
References
Gupta UC, Bhatia S, Garg A et al (2011) Phase 0 clinical trials in oncology new drug development. Perspect Clin Res 2:13–22. doi:10.4103/2229-3485.76285
Postel-Vinay S, Gomez-Roca C, Molife LR et al (2011) Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol Off J Am Soc Clin Oncol 29:1728–1735. doi:10.1200/JCO.2010.31.9236
Le Tourneau C, Delord J-P, Gonçalves A et al (2015) Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 16:1324–1334. doi:10.1016/S1470-2045(15)00188-6
Arkenau H-T, Barriuso J, Olmos D et al (2009) Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol Off J Am Soc Clin Oncol 27:2692–2696. doi:10.1200/JCO.2008.19.5081
Arkenau H-T, Olmos D, Ang JE et al (2008) Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer 98:1029–1033. doi:10.1038/sj.bjc.6604218
Benajiba L, Michot J-M, Baldini C et al (2017) Prognostic factors and outcome of patients with hematological malignancies in phase I trials: the Gustave Roussy scoring system. Anti-Cancer Drugs. doi:10.1097/CAD.0000000000000487
Protocol Development | CTEP. (2016) https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 19 Nov 2016
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5:649–655
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 25:579–586. doi:10.1200/JCO.2006.09.2403
Kumar A, Burger IA, Zhang Z et al (2016) Definition of bulky disease in early stage Hodgkin lymphoma in computed tomography era: prognostic significance of measurements in the coronal and transverse planes. Haematologica. doi:10.3324/haematol.2016.141846
Gallagher CJ, Gregory WM, Jones AE et al (1986) Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol Off J Am Soc Clin Oncol 4:1470–1480
Pfreundschuh M, Ho AD, Cavallin-Stahl E et al (2008) Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International trial group (MInT) study. Lancet Oncol 9:435–444. doi:10.1016/S1470-2045(08)70078-0
Postel-Vinay S, Collette L, Paoletti X, et al (2014) Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents--dose-limiting toxicity and toxicity assessment recommendation Group for Early Trials of targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. Eur J Cancer Oxf Engl 1990 50:2040–2049. doi:10.1016/j.ejca.2014.04.031
Sznol M (2000) (2010) reporting disease control rates or clinical benefit rates in early clinical trials of anticancer agents: useful endpoint or hype? Curr Opin Investig Drugs Lond Engl 11:1340–1341
Campo E, Swerdlow SH, Harris NL et al (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117:5019–5032. doi:10.1182/blood-2011-01-293050
Mallett S, Royston P, Dutton S et al (2010) Reporting methods in studies developing prognostic models in cancer: a review. BMC Med 8:20. doi:10.1186/1741-7015-8-20
Wheler J, Tsimberidou AM, Hong D, et al (2009) Survival of patients in a phase 1 clinic: the M. D Anderson Cancer Center experience Cancer 115:1091–1099. doi:10.1002/cncr.24018
Olmos D, A’hern RP, Marsoni S et al (2012) Patient selection for oncology phase I trials: a multi-institutional study of prognostic factors. J Clin Oncol Off J Am Soc Clin Oncol 30:996–1004. doi:10.1200/JCO.2010.34.5074
Italiano A, Massard C, Bahleda R et al (2008) Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol Off J Eur Soc Med Oncol ESMO 19:787–792. doi:10.1093/annonc/mdm548
Bachelot T, Ray-Coquard I, Catimel G et al (2000) Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol Off J Eur Soc Med Oncol 11:151–156
Booth CM, Calvert AH, Giaccone G et al (2008) Endpoints and other considerations in phase I studies of targeted anticancer therapy: recommendations from the task force on methodology for the development of innovative cancer therapies (MDICT). Eur J Cancer 44:19–24. doi:10.1016/j.ejca.2007.07.034
Postel-Vinay S (2015) Redefining dose-limiting toxicity. Clin Adv Hematol Oncol HO 13:87–89
Acknowledgements
The authors thank David Fraser (Biotech Communication SARL, Ploudalmézeau, France) for copy-editing assistance.
Author information
Authors and Affiliations
Contributions
JMM and LB equally contributed to this work. VR, LB, JCS, LF and JMM designed the study, collected and analyzed data, and wrote the manuscript. LF and XP analyzed data. LH reviewed CT data. CB, AJ, AH, SPV, BV, ZT, FB, CB, CB, AV, RB and AG collected data. SDB, CM, AD, DG, JL collected data and reviewed the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare no conflicts of interest with regard to this manuscript.
Funding
The work was funded by the Drug Development Department at the Gustave Roussy (Villejuif, France).
Ethical approval
All study procedures were approved by local independent ethics committee and were performed in accordance with the tenets of the 1964 Declaration of Helsinki and its amendments.
Informed consent
All patients had provided their written consent to inclusion of their data in the present study’s database.
Additional information
Highlights
• In the Phase I population with relapsed or refractory lymphoma, serum LDH, albumin levels and the histological type (tumour aggressiveness) were prognostic factors for overall survival.
• 39% of the patients experienced a first high-grade toxic event after the dose-limiting toxicity period, suggesting that the conventional concept of dose-limiting toxicity (designed for chemotherapy) should be redefined in the era of modern cancer therapies.
• The objective response and disease control rates were respectively 24% and 57% - suggesting that inclusion in an early-stage clinical trial is a valuable new treatment option in hard-to-treat relapsed or refractory lymphoma.
Electronic supplementary material
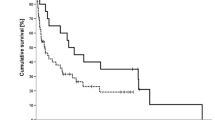
Figure 6
(supplemental material). Outcomes for patients with recurrent/refractory lymphoma participating in Phase I clinical trials, according to the histological type (aggressive non-Hodgkin lymphoma, indolent non-Hodgkin lymphoma, and Hodgkin lymphoma). ** Aggressive non-Hodgkin types included diffuse large B-cell lymphoma, peripheral T-cell lymphoma not otherwise specified, and mantle cell lymphoma. *** Indolent non-Hodgkin types included follicular lymphoma, lymphocytic lymphoma/B-cell chronic lymphocytic leukemia, Waldenstrom macroglobulinemia, and marginal zone lymphoma. (PPTX 138 kb)
Rights and permissions
About this article
Cite this article
Michot, JM., Benajiba, L., Faivre, L. et al. Outcomes and prognostic factors for relapsed or refractory lymphoma patients in phase I clinical trials. Invest New Drugs 36, 62–74 (2018). https://doi.org/10.1007/s10637-017-0480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0480-x