Summary
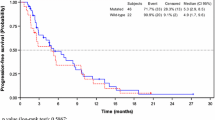
Background This study assessed the preliminary safety, pharmacokinetics (PK) and anti-tumor effects of aflibercept in combination with 5-fluorouracil, leucovorin and irinotecan (FOLFIRI) in Chinese patients with previously-treated advanced solid malignancies. Patients and Methods This open-label single-arm Phase I study conducted at two centers in China included adult (≥18 years) patients with metastatic or unresectable solid malignancies who had received ≥1 prior treatment. Patients received aflibercept 4 mg/kg IV on Day 1 followed by FOLFIRI over Days 1 and 2 every 2 weeks, and were assessed for safety, tumor response, PK parameters and immunogenicity. Post-hoc analyses included calculation of progression-free survival (PFS) for patients with colorectal cancer (CRC). Results A total of 20 patients were enrolled. The most common Grade 3/4 adverse events included neutropenia (35%), hypertension (30%), stomatitis (20%) and proteinuria (20%), and no anti-aflibercept antibodies were detected. Six patients achieved a partial response, and in 15 patients with CRC median PFS was 5.95 months (95% CI: 5.29–8.77). Free aflibercept remained in excess of VEGF-bound aflibercept for the majority of the study treatment duration. The mean free aflibercept values for Cmax (64.8 μg/mL) AUC (291 μg.day/mL), CL (0.92 L/day) and Vss (5.9 L) were similar to those measured in Caucasian patients. The addition of aflibercept did not influence the PK of the chemotherapy agents. Conclusion For Chinese patients with pre-treated advanced solid malignancies, 4 mg/kg of aflibercept in combination with FOLFIRI was well-tolerated, demonstrated preliminary anti-tumor activity and had a PK profile consistent with that in Caucasian patients.

Similar content being viewed by others
References
Ferrara N, Gerber Hp J, LeCouter F, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676
Folkman J (1997) Addressing tumor blood vessels. Nat Biotechnol 15(6):510
Coultas L, Chawengsaksophak J, Fau-Rossant K, Rossant J (2005) Endothelial cells and VEGF in vascular development. Nature 438(7070):937–945
Radinsky R, Ellis LM (1996) Molecular determinants in the biology of liver metastasis. Surg Oncol Clin N Am 5(2):215–229
Takahashi Y et al (1995) Expression of vascular endothelial growth factor and its receptor. KDR Cancer Res 55(18):3964–3968
Takebayashi Y et al (1996) Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer 78(2):226–231
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and. J Clin Oncol 23(5):1011–1027
Korpanty G, Smyth E (2012) Anti-VEGF strategies - from antibodies to tyrosine kinase inhibitors: background. Curr Pharm Des 18(19):2680–2701
Meadows KL, Hurwitz HI (2012) Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med 2(10). doi:10.1101/cshperspect.a006577.
Vasudev NS, Reynolds AR (2014) Anti-angiogenic therapy for cancer: current progress, unresolved questions and. Angiogenesis 17(3):471–494. doi:10.1007/s10456-014-9420-y
Fukasawa M, Korc M (2004) Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res 10(10):3327–3332
Holash J et al (2002) VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 99(17):11393–11398
Verheul HM et al (2007) Vascular endothelial growth factor trap blocks tumor growth, metastasis. Clin Cancer Res 13(14):4201–4208
Van Cutsem E et al (2013) Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur J Cancer 49(1):17–24. doi:10.1016/j.ejca.2012.07.007
Yoshino T et al (2013) A phase I study of intravenous aflibercept with FOLFIRI in Japanese patients with. Investig New Drugs 31(4):910–917. doi:10.1007/s10637-012-9895-6
Khayat D et al (2013) Intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours: results from the expansion cohort of a phase I study. Eur J Cancer 49(4):790–797. doi:10.1016/j.ejca.2012.10.012
Van Cutsem E et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30(28):3499–3506
Network, N.C.C (2016) Clinical practice guidelines in oncology: colon cancer (version 2.2016). 2006 January 1]; Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
Van Cutsem E, et al (2014) Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis. Ann Oncol 25(Suppl 3). doi:10.1093/annonc/mdu260
Lockhart AC et al (2010) Phase I study of intravenous vascular endothelial growth factor trap. J Clin Oncol 28(2):207–214. doi:10.1200/JCO.2009.22.9237
Tang PA et al (2012) Phase II clinical and pharmacokinetic study of aflibercept in patients with. Clin Cancer Res 18(21):6023–6031. doi:10.1158/1078-0432.CCR-11-3252
Verslype C et al (2008) Validation of the selected dose of aflibercept (VEGF trap) plus irinotecan, 5-fluorouracil, and leucovorin (I-LV5FU2) in a phase I clinical trial of patients (pts) with advanced solid tumors (STs): preliminary results. Journal of Clinical Oncology, 2008 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 26(15S (May 20 Supplement):14540
Acknowledgements
This study was sponsored by Sanofi (China). Editorial support for this manuscript was paid for by Sanofi and provided by Anne Wong at Adelphi Consultech.
Author contributions
All authors contributed to the study concept, design, conduct and interpretation of data. Ruihua Xu, Jianming Xu, Dongsheng Zhang and Rongrui Liu were responsible for data collection. Xing Sun provided statistical analysis and interpretation. All authors helped to draft and provide critical input into this manuscript, and approved the final version for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Yingxin Li, Xing Sun, Samira Ziti-Ljajic, Dongmei Shi and Nathalie Le bail are employees of Sanofi. The other authors have no further conflicts of interest to declare.
Funding
This study was sponsored by Sanofi (China). Editorial support for this manuscript was paid for by Sanofi and provided by Anne Wong at Adelphi Consultech.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
ESM 1
(DOCX 51 kb)
Rights and permissions
About this article
Cite this article
Xu, J., Li, Y., Sun, X. et al. A phase I and pharmacokinetic study of afilbercept with FOLFIRI: comparison of Chinese and Caucasian populations. Invest New Drugs 35, 463–470 (2017). https://doi.org/10.1007/s10637-016-0421-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0421-0