Summary
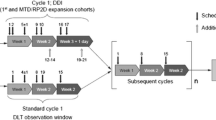
Background A wide variety of human cancers exhibit dysregulated c-Met activity that has implications in oncogenesis. Phosphorylation of c-Met results in activation of the PI3K/AKT/mTOR pathway. Combined blockade of c-Met and mTOR pathways has shown efficacy in preclinical studies. Tivantinib is a c-Met inhibitor and temsirolimus is a selective mTOR inhibitor. We aimed to determine the maximum tolerated dose (MTD) and the recommended phase II dose (RP2D), dose-limiting toxicities (DLT), adverse events (AEs), clinical activity and pharmacokinetic (PK) parameters of the combination. Methods This open-label phase I study used a 3 + 3 dose escalation design. Patients (pts) were treated with escalating doses of tivantinib (120–360 mg tablets orally twice daily) and temsirolimus (20 mg IV weekly) followed by dose expansion at the MTD. Separate cohorts were planned for extensive (normal) and poor tivantinib metabolizers based on CYP2C19 genotypes. Cycles were 28 days besides cycle 1 that was 35 days to allow for PK analysis. Results Twenty-nine pts. [median age 58 (range 28–77)] were enrolled (21 in dose escalation and 8 in dose expansion). All were extensive CYP2C19 metabolizers. The most common types of cancer were colorectal, ovarian and non-small cell lung. Sixteen out of 21 and 6 out of 8 pts. were evaluable for DLT evaluation per protocol in the dose escalation and dose expansion phases, respectively. Pts remained on study for a median of 71 days (range 18–296). The MTD and RP2D was tivantinib 240 mg twice daily and temsirolimus 20 mg weekly. DLTs included grade (gr) 4 neutropenia (2 pts.; 1 with gr 3 febrile neutropenia), gr 3 abdominal pain (1 pt) and gr 2 mucositis resulting in inadequate drug delivery. The most common treatment related AEs grade ≥ 2 included: anemia (gr 2 in 9 pts., gr 3 in 3 pts), fatigue (gr 2 in 10 pts), anorexia (gr 2 in 9 pts), hypoalbuminemia (gr 2 in 6 pts., gr 3 in 2 pts), hypophosphatemia (gr 2 in 2 pts., gr 3 in 5 pts) and nausea (gr 2 in 6 pts., gr 3 in 1 pt). One pt. with ovarian cancer had a confirmed partial response and remained on study for 10 months, a second patient with ovarian cancer had stable disease and remained on study for 6 months and a third pt. with squamous cell carcinoma of the tongue had stable disease and remained on study for 7 months. Pharmacokinetic analysis showed that there is no interaction in the plasma concentrations between tivantinib and temsirolimus. Conclusions The combination of tivantinib with temsirolimus appears to be well tolerated with evidence of clinical activity.

Similar content being viewed by others
References
Robinson DR, YM W, Lin SF (2000) The protein tyrosine kinase family of the human genome. Oncogene 19(49):5548–5557
Zwick E, Bange J, Ullrich A (2001) Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer 8(3):161–173
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12):915–925
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G, Targeting MET (2012) In cancer: rationale and progress. Nat Rev Cancer 12(2):89–103
Schmidt L, Duh FM, Chen F et al (1997) Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16(1):68–73
Christensen JG, Schreck R, Burrows J et al (2003) A selective small molecule inhibitor of c-met kinase inhibits c-met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 63(21):7345–7355
Furge KA, Zhang YW, Vande Woude GF (2000) Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene 19(49):5582–5589
Munshi N, Jeay S, Li Y et al (2010) ARQ 197, a novel and selective inhibitor of the human c-met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther 9(6):1544–1553
Investigator’s Brochure. ARQ 197 (Version 5.0 O, 2010). Daiichi Sankyo pharma development, Edison, NJ.
Yap TA, Olmos D, Brunetto AT et al (2011) Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol 29(10):1271–1279
Rosen LS, Senzer N, Mekhail T et al (2011) A phase I dose-escalation study of tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res 17(24):7754–7764
Yamamoto N, Murakami H, Hayashi H et al (2013) CYP2C19 genotype-based phase I studies of a c-met inhibitor tivantinib in combination with erlotinib, in advanced/metastatic non-small cell lung cancer. Br J Cancer 109(11):2803–2809
Wagner AJ, Goldberg JM, Dubois SG et al (2012) Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer 118(23):5894–5902
Santoro A, Rimassa L, Borbath I et al (2013) Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 14(1):55–63
Tolaney SM, Tan S, Guo H et al (2015) Phase II study of tivantinib (ARQ 197) in patients with metastatic triple-negative breast cancer. Investig New Drugs 33(5):1108–1114
Dorkhom SJ et al. (2012) Phase II study of the c-Met inhibitor ARQ 197 (tivantinib) in patients with relapsed multiple myeloma (https://ash.confex.com/ash/2012/webprogram/Paper53995.html)
Sequist LV, von Pawel J, Garmey EG et al (2011) Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 29(24):3307–3315
Goldman JW, Laux I, Chai F et al (2012) Phase 1 dose-escalation trial evaluating the combination of the selective MET (mesenchymal-epithelial transition factor) inhibitor tivantinib (ARQ 197) plus erlotinib. Cancer 118(23):5903–5911
Yoshioka H, Azuma K, Yamamoto N et al (2015) A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol 26(10):2066–2072
Scagliotti G, von Pawel J, Novello S et al (2015) Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 33(24):2667–2674
Pant S, Saleh M, Bendell J et al (2014) A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol 25(7):1416–1421
Puzanov I, Sosman J, Santoro A et al (2015) Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Investig New Drugs 33(1):159–168
Eng C, Bessudo A, Hart LL et al (2016) A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int J Cancer 139(1):177–186
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281
Boni JP, Hug B, Leister C, Sonnichsen D (2009) Intravenous temsirolimus in cancer patients: clinical pharmacology and dosing considerations. Semin Oncol 36(Suppl 3):S18–S25
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Raymond E, Alexandre J, Faivre S et al (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22(12):2336–2347
Garajová I, Giovannetti E, Biasco G, Peters GJ (2015) C-met as a target for personalized therapy. Transl Oncogenomics 7(Suppl 1):13–31
Ma PC, Schaefer E, Christensen JG, Salgia RA (2005) Selective small molecule c-MET inhibitor, PHA665752, cooperates with rapamycin. Clin Cancer Res 11(6):2312–2319
Chang SM, Kuhn J, Wen P et al (2004) Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Investig New Drugs 22(4):427–435
Rodriguez-Antona C, Ingelman-Sundberg M (2006) Cytochrome P450 pharmacogenetics and cancer. Oncogene 25(11):1679–1691
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41(12):913–958
Basilico C, Pennacchietti S, Vigna E et al (2013) Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin Cancer Res 19(9):2381–2392
Katayama R, Aoyama A, Yamori T et al (2013) Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res 73(10):3087–3096
Acknowledgements
The investigators gratefully acknowledge the patients and families who participated in this study. Funding was provided by the NCI Cancer Center Support Grant P30 CA014520 to University of Wisconsin Carbone Cancer Center and NCI U01CA062491-19S1 Early Clinical Trials of Anti-Cancer Agents with Phase I Emphasis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflict of interest.
Funding
Funding was provided by the NCI Cancer Center Support Grant P30 CA014520 to University of Wisconsin Carbone Cancer Center and NCI U01CA062491-19S1 Early Clinical Trials of Anti-Cancer Agents with Phase I Emphasis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kyriakopoulos, C.E., Braden, A.M., Kolesar, J.M. et al. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Invest New Drugs 35, 290–297 (2017). https://doi.org/10.1007/s10637-016-0418-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0418-8