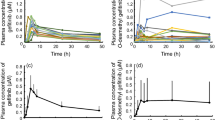
Summary
Background Older non-small cell lung cancer (NSCLC) patients under erlotinib are reported to experience more acute toxicity. We hypothesized that modifications in erlotinib pharmacokinetics might explain this observation. Methods A monocentric prospective clinico-pharmacological study included stage IIIb/IV NSCLC consecutive pts. treated with erlotinib. The plasma concentration of erlotinib (Ce) was measured at steady state on day 15. We studied the relationship between age > 75 years, and Ce, using the Mann-Whitney U test and with the occurrence of acute toxicity, using a Fisher’s test. Results A total of 53 pts. were analyzed. Median age was 68 years (31–83), 56 % were female. All pts. > 75 years experienced toxicity: all grade acute adverse events were 1.6 fold more frequent (100 % vs 61 %; OR 95 % CI [1.9-INF]; p = 0.003). At day 15, Ce increased with age. Over 75 years old, the mean Ce was 1.5 fold higher: 2091 ng/mL (95 % CI [1476; 2706]) vs 1359 (95 % CI [1029; 1689]; p = 0.024). In pts. over 80 years old, the mean Ce was doubled: 2729 (95 % CI [1961; 3497]) vs 1358 ng/mL (95 % CI [1070; 1646]; p = 0.0019). Reduced lean body mass over 75 years (median 36.6 kg versus 49.1 kg) might account for these differences. Finally, the risk of early erlotinib discontinuation was increased by 11 in older pts. (33 % vs 3 % OR 17.2; 95 % CI [1.7; 892.5] p = .005). Conclusion The risk of overexposure to erlotinib increases with age. Reduced lean body mass may explain erlotinib pharmacokinetics and excessive acute toxicity in the elderly.


Similar content being viewed by others
References
Zeng C, Wen W, Morgans AK, Pao W, Shu X-O, Zheng W (2015) Disparities by race, age, and sex in the improvement of survival for major cancers: results from the national cancer institute surveillance, epidemiology, and end results (SEER) program in United States, 1990 to 2010. JAMA Oncol 1(1):88–96
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L (2005) Erlotinib in previously treated non–small-cell lung cancer. N. Engl J Med 353(2):123–132
Chen Y-M, Lai C-H, Rau K-M, Huang C-H, Chang H-C, Chao T-Y, Tseng C-C, Fang W-F, Chen Y-C, Chung Y-H, Wang Y-H, Su M-C, Huang K-T, Liu S-F, Chen H-C, Chang Y-C, Chang Y-P, Wang C-C, Lin M-C Advanced non-Small cell lung cancer patients at the extremes of age in the era of epidermal growth factor receptor tyrosine kinase inhibitors. Lung Cancer 98:99–105
Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA (2008) Erlotinib for advanced non–small-cell lung cancer in the elderly: an analysis of the national cancer institute of canada clinical trials group study BR.21. J. Clin. Oncol. 26(14):2350–2357
Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, Skarin AT, Meyerson M, Holmes AJ, Borras AM, Freidlin B, Ostler PA, Lucca J, Lynch TJ, Johnson BE, Jänne PA (2007) Phase II clinical trial of chemotherapy-Naïve patients ≥70 Years of age treated with erlotinib for advanced non–small-cell lung cancer. J. Clin Oncol 25(7):760–766
Yamada K, Azuma K, Takeshita M, Uchino J, Nishida C, Suestsugu T et al (2016) Phase II trial of erlotinib in elderly patients with previously treated non-small cell lung cancer: results of the lung oncology group in Kyushu (LOGiK-0802). Anticancer Res. 36(6):2881–2887
Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, Ferrante K, Hoff DDV, Silberman S, Rowinsky EK (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J. Clin Oncol 19(13):3267–3279
Lu J-F, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, Lum BL (2006) Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non–small cell lung cancer. Clin. Pharmacol Ther 80(2):136–145
Sjøblom B, Grønberg BH, Benth JŠ, Baracos VE, Fløtten Ø, Hjermstad MJ, Aass N, Jordhøy M Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer 90(1):85–91
Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB (2010) Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 21(8):1594–1598
Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, Ropert S, Delongchamps NB, Zerbib M, Goldwasser F (2013) Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108(5):1034–1041
Mir O, Blanchet B, Goldwasser F (2011) Drug-induced effects on erlotinib metabolism. N. Engl J Med 365(4):379–380
Zhen Y, Thomas-Schoemann A, Sakji L, Boudou-Rouquette P, Dupin N, Mortier L, Vidal M, Goldwasser F, Blanchet B (2013) An HPLC-UV method for the simultaneous quantification of vemurafenib and erlotinib in plasma from cancer patients. J. Chromatogr B 928:93–97
Foo J, Chmielecki J, Pao W, Michor F (2012) Effects of pharmacokinetic processes and varied dosing schedules on the dynamics of acquired resistance to erlotinib in EGFR-mutant lung cancer. J. Thorac Oncol 7(10):1583–1593
Yoshioka H, Komuta K, Imamura F, Kudoh S, Seki A, Fukuoka M Efficacy and safety of erlotinib in elderly patients in the phase IV POLARSTAR surveillance study of Japanese patients with non-small-cell lung cancer. Lung Cancer 86(2):201–206
Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, Wu G, Liu W, Liao G, Cai K, Chen L, Zheng M, Yu P, Wang X, Liu Y, Guo Q, Nie L, Liu J, Han X (2015) Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology – Mainland China subset analysis of the PIONEER study. PLoS ONE 10(11):e0143515
Mir O, Coriat R, Blanchet B, Durand J-P, Boudou-Rouquette P, Michels J, Ropert S, Vidal M, Pol S, Chaussade S, Goldwasser F (2012) Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 7(5)
Narjoz C, Cessot A, Thomas-Schoemann A, Golmard J (2015) Role of the lean body mass and of pharmacogenetic variants on the pharmacokinetics and pharmacodynamics of sunitinib in cancer patients. Investig New Drugs 33:257–268
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pascaline Boudou-Rouquette has received honoraria from Roche and Novartis, outside the submitted work. Pr Goldwasser reports personal fees from Bayer, Roche, Fresenius, Sanofi-Avantis, Astra-Zeneca and Boeringher, outside the submitted work. Jeanne Chapron reported fees from Novartis and Astra-Zeneca outside the submitted work. Olivier Huillard has received personal fees from Bayer, MSD and Astellas.
Funding
The work was supported by the Department of Medical Oncology, Paris Descartes University, Cochin - Port Royal Hospital, AP-HP, Paris, France and the Department of Pharmacocinétique et pharmacochimie, Paris Descartes University, Cochin - Port Royal Hospital, AP-HP, Paris, France.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with 1964 Helsinki declaration and its later amendments or comparable standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bigot, F., Boudou-Rouquette, P., Arrondeau, J. et al. Erlotinib pharmacokinetics: a critical parameter influencing acute toxicity in elderly patients over 75 years-old. Invest New Drugs 35, 242–246 (2017). https://doi.org/10.1007/s10637-016-0400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0400-5