Summary
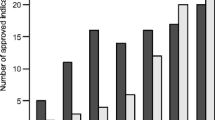
To reduce the delay in marketing authorization of drugs in Japan, four Japanese national projects were instituted. We examined all oncologic drugs for adult patients approved or discussed through these schemes, for the first time. All the data are publicly available. In total, 197 applications/demands (181 indications and 16 dosages/uses) were collected. As of December 31, 2015, 64 indications and 10 dosages/uses were approved as off-label drugs through these schemes without conducting additional registration trials in Japan. Furthermore, 46 indications and two dosages/uses were approved after registration trials in Japan requested by the national scheme councils. Regarding the following 23 indications of the 197 applications/demands, registration trials in Japan were commenced after the national scheme council’s request: 17 hematological malignancies and six orphan solid tumors. Moreover, 54 indications and three dosages/uses, for which demands were submitted, were regarded as not a high medical priority by the national scheme council. Regarding two hematological malignancy indications, the dosage approved in foreign countries was intolerable for the Japanese patients in Japanese registration trials and this stopped the clinical development in Japan. Our analysis showed that 110 indications and 12 dosages/uses were approved in Japan through these schemes. These national projects have provided numerous therapeutic options for Japanese patients and may be meaningful for promoting clinical development and regulatory approval especially in orphan diseases in countries other than Japan.
Similar content being viewed by others
References
Nagai S, Ozawa K (2016) Clinical trial designs to obtain marketing authorization of drugs for haematological malignancy in Japan, the EU and the US. Br J Haematol 174:249–254
Ito Y, Narimatsu H, Fukui T, Fukao A, Yoshioka T (2013) Critical review of ‘Public domain application’: a flexible drug approval system in Japan. Ann Oncol 24:1297–1305
Tenhunen O (2013) How to assess assessments? Ann Oncol 24:1138–1140
Narimatsu H, Oiso G, Ono S, Kami M (2009) Critical review of the determination process by the Japanese reviewing authority in approving the additional efficacy of fludarabine phosphate. J Clin Oncol 27:e236–e238
Shimazawa R, Ikeda M (2012) Japanese regulatory system for approval of off-label drug use: evaluation of safety and effectiveness in literature-based applications. Clin Ther 34:2104–2116
MHLW (2016) website Handling on off-label use Available at http://www.mhlw.go.jp/shingi/2005/01/dl/s0124-9h1.pdf [in Japanese]. Accessed July 31
MHLW (2016) website Current status of demands in the Council Available at http://www.mhlw.go.jp/stf/shingi/2r9852000002ghwn-att/2r9852000002gih9.pdf [in Japanese]. Accessed July 31
MHLW (2016) website SAKIGAKE designation Available at http://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000089800.pdf [in Japanese]. Accessed July 31
MHLW (2016) website The Council on Combination Therapy for Cancer Available at http://www.mhlw.go.jp/shingi/2004/05/s0521-5.html [in Japanese]. Accessed July 31
MHLW (2016) website The Council on Unapproved Drugs Available at http://www.mhlw.go.jp/stf/shingi/other-iyaku.html?tid=128702 [in Japanese]. Accessed July 31
MHLW (2016) website The Council on the Development Promotion Scheme for unapproved and off-Label Drugs Available at http://www.mhlw.go.jp/stf/shingi/other-iyaku.html?tid=128701 [in Japanese]. Accessed July 31
MHLW (2016) website Minutes of The Council on Combination Therapy for Cancer Available at http://www.mhlw.go.jp/shingi/2004/08/s0825-4.html [in Japanese]. Accessed July 31
PMDA (2016) website Search for information on approved drugs Available at http://www.pmda.go.jp/PmdaSearch/iyakuSearch/ [in Japanese]. Accessed July 31
Borg JJ, Laslop A, Pani L, Maciulaitis R, Melchiorri D (2014) Reflections on Decisions Made on the Well-Established Use of Medicinal Products by EU Regulators and the ECJ. Sci Pharm 82:541–554
Guidance for Industry Applications Covered by Section 505(b) (2) (2016) Draft Guidance Available at http://www.fda.gov/downloads/Drugs/.../Guidances/ucm079345.pdf Accessed July 31
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 15 K20967.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Nagai was paid for consulting or an advisory role by Takara Bio Inc. Dr. Ozawa received grant or research funding from Takara Bio Inc. and Sumitomo Dainippon Pharma Co. Ltd. Dr. Ozawa was paid for consulting or an advisory role by JCR Pharmaceutical Inc., and Celgene Japan.
Authorship Contributions
SN designed the study and collected, analyzed, and interpreted the data, and wrote the manuscript. KO supervised the research and wrote the manuscript.
Rights and permissions
About this article
Cite this article
Nagai, S., Ozawa, K. Comprehensive analysis of clinical development and regulatory submission promotion schemes for oncologic drugs as the Japanese national projects. Invest New Drugs 34, 777–791 (2016). https://doi.org/10.1007/s10637-016-0380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0380-5