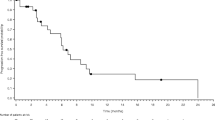
Abstract
Background Axitinib is an oral, potent, small molecule tyrosine kinase inhibitor with selective inhibition of VEGFR 1,2, 3, as well as inhibition of potential downstream effectors of the EGFR pathway. Given the upregulation of EGFR and VEGFR in head and neck squamous cell carcinoma, treatment with axitinib holds promise as a rational targeted therapy. Patients and Methods Patients with unresectable, recurrent or metastatic head and neck squamous cell carcinoma were included in this open label, single arm, phase II trial. Primary endpoint was 6 month progression free survival. All patients received single agent axitinib with planned dose escalation based on tolerability. A planned interim efficacy analysis was performed after enrollment of 30 patients. Results Forty-two patients were registered, 30 were evaluable. While treatment was well-tolerated with no severe bleeding events, only 19 patients were able to achieve full planned dose. The best overall response rate was 6.7 % (two partial responses) with a disease control rate of 76.7 %. Median progression free survival was 3.7 months (95 % Confidence Interval (CI): 3.5–5.7) and overall survival was 10.9 months (95 % CI: 6.4–17.8). Exploratory analysis demonstrated that patients with a smaller sum of diameter of target lesions experienced improved response rates, and better progression-free and overall survival. Conclusion Treatment with single agent axitinib should be considered due to acceptable toxicity profile and favorable median overall survival compared to standard therapies.



Similar content being viewed by others
References
Hutson TE, Lesovoy V, Al-Shukri S, et al (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14:1287–1294
Motzer RJ, Escudier B, Tomczak P, et al (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14:552–562
Locati LD, Licitra L, Agate L, et al (2014) Treatment of advanced thyroid cancer with axitinib: phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer 120:2694–2703
Cohen EE, Tortorici M, Kim S, et al (2014) A phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother Pharmacol 74:1261–1270
Hu-Lowe DD, Zou HY, Grazzini ML, et al (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14:7272–7283
Schiller JH, Larson T, Ou SH, et al (2009) Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol 27:3836–3841
Furnival GM, Wilson RW (1974) Regressions by leaps and bounds. Technometrics 16:499–511
Cohen EE, Kane MA, List MA, et al (2005) Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 11:8418–8424
Cohen MH, Williams GA, Sridhara R, et al (2003) FDA drug approval summary: gefitinib (ZD1839) (iressa) tablets. Oncologist 8:303–306
Stewart JS, Cohen EE, Licitra L, et al (2009) Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 27:1864–1871
Soulieres D, Senzer NN, Vokes EE, et al (2004) Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22:77–85
Williamson SK, Moon J, Huang CH, et al (2010) Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: southwest oncology group study S0420. J Clin Oncol 28:3330–3335
Gilbert J, Schell MJ, Zhao X, et al (2015) A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol 51:376–382
Kim HS, Kwon HJ, Jung I, et al (2015) Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin Cancer Res 21:544–552
Bechtold RE, Chen MY, Stanton CA, et al (2003) Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with gleevec. Abdom Imaging 28:808–814
Linton KM, Taylor MB, Radford JA (2006) Response evaluation in gastrointestinal stromal tumours treated with imatinib: misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol 79:e40–e44
Benjamin RS, Choi H, Macapinlac HA, et al (2007) We should desist using RECIST, at least in GIST. J Clin Oncol 25:1760–1764
Choi H, Charnsangavej C, Faria SC, et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759
Therasse P, Arbuck SG, Eisenhauer EA, et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92:205–216
Goh V, Ganeshan B, Nathan P, et al (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261:165–171
Kambadakone A, Yoon SS, Kim TM, et al (2015) CT perfusion as an imaging biomarker in monitoring response to neoadjuvant bevacizumab and radiation in soft-tissue sarcomas: comparison with tumor morphology, circulating and tumor biomarkers, and gene expression. AJR Am J Roentgenol 204:W11–W18
Fakhry C, Westra WH, Li S, et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269
Chaturvedi AK, Engels EA, Pfeiffer RM, et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301
Ang KK, Harris J, Wheeler R, et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Huang H, Zhang B, Chen W, et al (2012) Human papillomavirus infection and prognostic predictors in patients with oropharyngeal squamous cell carcinoma. Asian Pac J Cancer Prev 13:891–896
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14:6735–6741
Petreaca ML, Yao M, Liu Y, et al (2007) Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell 18:5014–5023
Gyanchandani R, Sano D, Ortega Alves MV, et al (2013) Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol 49:761–770
Le QT, Fisher R, Oliner KS, et al (2012) Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clin Cancer Res 18:1798–1807
Rischin D, Peters LJ, O'Sullivan B, et al (2010) Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the trans-Tasman radiation oncology group. J Clin Oncol 28:2989–2995
Acknowledgments
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swiecicki, P.L., Zhao, L., Belile, E. et al. A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Invest New Drugs 33, 1248–1256 (2015). https://doi.org/10.1007/s10637-015-0293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0293-8