Summary
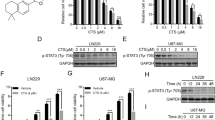
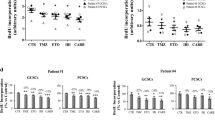
Background Glioblastoma (GBM), the most common and aggressive primary brain tumor, is characterized by excessive brain infiltration which prevents the complete surgical resection. These tumors also display treatment non-compliance and responses to standard therapy are invariably transient; consequently, the prognosis barely exceeds 14 months and recurrence is inevitable. Accordingly, several new treatment strategies have been studied. One such option is the use of chloroquine (CQ), a lysosomotropic weak base and renowned antimalarial drug, that has shown promising results in several pre-clinical studies. In this paper, we investigate the efficiency of CQ to hinder the malignant phenotype of GBM, namely extensive proliferation, invasion and radio-resistance. Results In cell cycle analysis, proliferation assays and immunofluorescence, CQ treatments halved proliferation of primary cultures from GBM specimens and GBM cell lines (U-373 MG et U-87 MG). Gelatin zymography and MatrigelTM-coated transwell invasion assays also revealed a 50 % CQ induced inhibition of MMP-2 activity and GBM invasion. Concomitant treatment with CQ and radiation also radiosensitized GBM cells as shown by an accumulation in the G2/M phase, increased cell death and reduced clonogenic formation. Moreover, radiation-induced invasion was considerably restrained by CQ. We also observe that these effects are owed to CQ-induced inhibition of TGF-β secretion and signaling pathway, a predominant growth factor in GBM progression. Conclusion These results suggest that CQ, alone or as an adjuvant therapeutic, could be used to inhibit the GBM malignant phenotype and could benefit GBM afflicted patients.






Similar content being viewed by others
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncology 14(Suppl 5):v1–v49
Fritz A, Percy C, Jack A, Shanmugaratnam K, Parkin DM, Whelan S (2012) International Classification of Diseases for Oncology, 3rd edn. Publication of the World Health Organization 1–250
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff R-O, Groups EOFRATOCBTARO, National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5 years analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Beier D, Schulz JB, Beier CP (2011) Chemoresistance of glioblastoma cancer stem cells--much more complex than expected. Mol Cancer 10:128
Bleau A-M, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC (2009) PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4:226–235
Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu I-M, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Hartigan J, Smith DR, Strausberg RL, Marie SKN, Shinjo SMO, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, Groups EOFRATOCBTAR, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA (2014) Bevacizumab and glioblastoma: Scientific review, newly reported updates, and ongoing controversies. Cancer. doi:10.1002/cncr.28935
Kilic T, Alberta JA, Zdunek PR, Acar M, Iannarelli P, O’Reilly T, Buchdunger E, Black PM, Stiles CD (2000) Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res 60:5143–5150
Reardon DA, Cheresh D (2012) Cilengitide: a prototypic integrin inhibitor for the treatment of glioblastoma and other malignancies. Genes Cancer 2:1159–1165. doi:10.1177/1947601912450586
Wen PY, Lee EQ, Reardon DA, Ligon KL, Alfred Yung WK (2012) Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro-Oncology 14:819–829. doi:10.1093/neuonc/nos117
Jiang P, Mukthavaram R, Mukthavavam R, Chao Y, Bharati IS, Fogal V, Pastorino S, Cong X, Nomura N, Gallagher M, Abbasi T, Vali S, Pingle SC, Makale M, Kesari S (2014) Novel anti-glioblastoma agents and therapeutic combinations identified from a collection of FDA approved drugs. J Transl Med 12:13. doi:10.1186/1479-5876-12-13
Rahman R, Hempfling K, Norden AD, Reardon DA, Nayak L, Rinne ML, Beroukhim R, Doherty L, Ruland S, Rai A, Rifenburg J, LaFrankie D, Alexander BM, Huang RY, Wen PY, Lee EQ (2014) Retrospective study of carmustine or lomustine with Bevacizumab in recurrent glioblastoma patients who have failed prior Bevacizumab. Neuro-Oncology 16:1523–1529. doi:10.1093/neuonc/nou118
Briceño E, Calderon A, Sotelo J (2007) Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg Neurol 67:388–391. doi:10.1016/j.surneu.2006.08.080
Kim EL, Wüstenberg R, Rübsam A, Schmitz-Salue C, Warnecke G, Bücker E-M, Pettkus N, Speidel D, Rohde V, Schulz-Schaeffer W, Deppert W, Giese A (2010) Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncology 12:389–400. doi:10.1093/neuonc/nop046
Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK (2012) Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res 72:1773–1783. doi:10.1158/0008-5472.CAN-11-3831
Manic G, Obrist F, Kroemer G, Vitale I (2014) Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell. doi:10.4161/mco.29911
Clark DA, Coker R (1998) Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol 30:293–298
Dubois CM, Blanchette F, Laprise M-H, Leduc R, Grondin F, Seidah NG (2001) Evidence that furin is an authentic transforming growth factor-β1-converting enzyme. Am J Pathol 158:305–316
Dubois CM, Laprise M-H, Blanchette F, Gentry LE, Leduc R (1995) Processing of transforming growth factor b1 precusor by human furin convertase. J Biol Chem 270:10618–10624
Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, Weller M (2004) RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res 64:7596–7603
Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH (2012) Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res 72:4119–4129
Pepper MS (1997) Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8:21–43
Seoane J, Le H-V, Shen L, Anderson SA, Massagué J (2004) Integration of smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117:211–223
Wick W, Platten M, Weller M (2001) Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neuro-Oncol 53:177–185
Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A (1992) Differential expression of transforming growth factor-beta 1, −beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol 148:1404–1410
Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I, Salford LG, Sjögren HO, Widegren B (2000) Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer 89:251–258
Roy L-O, Poirier M-B, Fortin D (2015) Transforming growth factor-beta and its implication in the malignancy of gliomas. Target Oncol 10:1–14. doi:10.1007/s11523-014-0308-y
Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, Prieto-SAnchez RM, Barba I, Martínez-Sáez E, Prudkin L, Cuartas I, Raventós C, Martínez-Ricarte F, Poca MA, GarcIa-Dorado D, Lahn MM, Yingling JM, Rodón J, Sahuquillo J, Baselga J, Seoane J (2010) TGF-beta receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell 18:655–668. doi:10.1016/j.ccr.2010.10.023
Jennings MT, Pietenpol JA (1998) The role of transforming growth factor beta in glioma progression. J Neuro-Oncol 36:123–140
Peñuelas S, Anido J, Prieto-SAnchez RM, Folch G, Barba I, Cuartas I, GarcIa-Dorado D, Poca MA, Sahuquillo J, Baselga J, Seoane J (2009) TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 15:315–327. doi:10.1016/j.ccr.2009.02.011
Byfield SD, Major C, Laping NJ (2004) SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 65:744–752
Schlingensiepen R, Goldbrunner M, Szyrach MNI, Stauder G, Jachimczak P, Bogdahn U, Schulmeyer F, Hau P, Schlingensiepen K-H (2005) Intracerebral and intrathecal infusion of the TGF-β2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides 15:94–104
Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, McDonald HA, Harper J, Lonning S, Okada H (2009) Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res 15:6551–6559
Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ying Ma J, Almirez R, Mangadu R, Liu Y-W, Platten M, Herrlinger U, Murphy A, Wong DH, Wick W, Higgins LS, Weller M (2004) SD-208, a novel transforming growth factor receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 64:7954–7961
Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR (1999) Gelatinase-a (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer 79:1828–1835. doi:10.1038/sj.bjc.6690291
Desmarais G, Fortin D, Bujold R, Wagner R, Mathieu D, Paquette B (2012) Infiltration of glioma cells in brain parenchyma stimulated by radiation in the F98/fischer rat model. Int J Radiat Biol 88:565–574
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. doi:10.1056/NEJMra0708126
Duguay SJ, Jin Y, Stein J, Duguay AN, Gardner P, Steiner DF (1998) Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J Biol Chem 273:18443–18451
Rockwell NC, Krysan DJ, Komiyama T, Fuller RS (2002) Precursor processing by Kex2/furin proteases. Chem Rev 102:4525–4548. doi:10.1021/cr010168i
Wick W, Wild-Bode C, Frank B, Weller M (2004) BCL-2-induced glioma cell invasiveness depends on furin-like proteases. J Neurochem 91:1275–1283. doi:10.1111/j.1471-4159.2004.02806.x
Ridley RG (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686–693. doi:10.1038/415686a
Shujatullah F, Khan HM, Khatoon A, Khan PA, Ashfaq M (2012) In vitro chloroquine resistance in plasmodium falciparum isolates from tertiary care hospital. Malar Res Treat 2012:1–4. doi:10.1016/S1473-3099(10)70214-0
Chan K-S, Koh C-G, Li H-Y (2012) Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 3, e411. doi:10.1038/cddis.2012.148
Firat E, Weyerbrock A, Gaedicke S, Grosu A-L, Niedermann G (2012) Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote γ-irradiation-induced cell death in primary stem-like glioma cells. PLoS ONE 7, e47357. doi:10.1371/journal.pone.0047357
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11:407–420. doi:10.1016/j.ccr.2007.04.001
Geng Y, Kohli L, Klocke BJ, Roth KA (2010) Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro-Oncology 12:473–481. doi:10.1093/neuonc/nop048
Dix AR, Brooks WH, Roszman TL, Morford LA (1999) Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol 100:216–232
Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC (1991) Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8 + T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol 146:3289–3297
Gustafsson LL, Walker O, Alván G, Beermann B, Estevez F, Gleisner L, Lindström B, Sjöqvist F (1983) Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol 15:471–479
Marques MM, Costa MRF, Santana Filho FS, Vieira JLF, Nascimento MTS, Brasil LW, Nogueira F, Silveira H, Reyes-Lecca RC, Monteiro WM, Lacerda MVG, Alecrim MGC (2013) Plasmodium vivax chloroquine resistance and anemia in the western Brazilian amazon. Antimicrob Agents Chemother 58:342–347. doi:10.1128/AAC.02279-12
Pukrittayakamee S, Tarning J, Jittamala P, Charunwatthana P, Lawpoolsri S, Lee SJ, Hanpithakpong W, Hanboonkunupakarn B, Day NPJ, Ashley EA, White NJ (2014) Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob Agents Chemother 58:3354–3359. doi:10.1128/AAC.02794-13
Rengelshausen J, Burhenne JR, Fröhlich M, Tayrouz Y, Singh SK, Riedel K-D, Müller O, Hoppe-Tichy T, Haefeli WE, Mikus G, Walter-Sack I (2004) Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur J Clin Pharmacol 60:709–715. doi:10.1007/s00228-004-0818-0
Broccatelli F, Larregieu CA, Cruciani G, Oprea TI, Benet LZ (2012) Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev 64:95–109. doi:10.1016/j.addr.2011.12.008
Acknowledgments
We thank Pr Claire Dubois for kindly providing the 2× SYBR Green qPCR Mastermix and PAI-1 primers as well as for helpful discussions. We also thank Pr. Jean-François Beaulieu for the gift of the immunofluorescence antibodies and Dr. Leonid Volkov for his technical assistance during flow cytometry experiments. All culture media were generously provided by Wisent Bioproducts.
Grant support
This work was supported by the National Bank research chair on the treatment of brain tumors as well as by the Fondation du CHUS and the Fondation de l’université de Sherbrooke.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, LO., Poirier, MB. & Fortin, D. Chloroquine inhibits the malignant phenotype of glioblastoma partially by suppressing TGF-beta. Invest New Drugs 33, 1020–1031 (2015). https://doi.org/10.1007/s10637-015-0275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0275-x