Summary
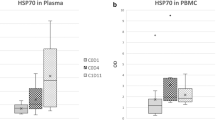
Inhibition of heat shock 90 (Hsp90) molecular chaperones allows targeting of multiple proteins involved in tumorigenesis. We investigated the safety, recommended phase 2 dose (RP2D), and pharmacokinetic and pharmacodynamic profile of onalespib (AT13387), a potent synthetic Hsp90 inhibitor, administered on days 1, 2, 8, 9, 15, and 16 of 28 day cycles (QDx2/week) in a phase I trial. This study followed an accelerated titration design with a starting dose of 20 mg/m2/dose and a standard 3 + 3 dose escalation design for dose level 4 (120 mg/m2/dose) and above. Additional patients were enrolled at the RP2D with mandatory paired tumor biopsies to assess modulation of 210 client proteins using reverse phase protein array analysis. Thirty-one patients were treated; RP2D was established at 160 mg/m2/dose on the QDx2/week schedule. Common toxicities were gastrointestinal, hepatic, and hematologic. Pharmacokinetic profile was linear and plasma levels increased proportionally with dose (T½ ~8 h). No responses were observed; eight patients had stable disease for > 2 cycles with one patient remaining on study for 6 cycles. Target engagement was demonstrated by transcriptional upregulation of Hsp70 and Hsp27 in PBMCs. Statistically significant modulation of client proteins was not achieved in the 9 paired tumor biopsies evaluated; however, hierarchical clustering revealed two subgroups of patients with differential patterns of protein expression. Further combination studies are needed in order to target prospective driver oncoproteins.




Similar content being viewed by others
References
Pratt WB (1998) The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 217(4):420–434
Sidera K, Patsavoudi E (2008) Extracellular HSP90: conquering the cell surface. Cell Cycle 7(11):1564–1568
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5(10):761–772. doi:10.1038/nrc1716
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549. doi:10.1038/nrc2887
Neckers L, Schulte TW, Mimnaugh E (1999) Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Investig New Drugs 17(4):361–373
Usmani SZ, Bona R, Li Z (2009) 17 AAG for HSP90 inhibition in cancer–from bench to bedside. Curr Mol Med 9(5):654–664
Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA, Horneffer YR, Juwara L, Melillo G, Ivy SP, Merino M, Neckers L, Steeg PS, Conley BA, Giaccone G, Doroshow JH, Murgo AJ (2010) Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur J Cancer 46(2):340–347. doi:10.1016/j.ejca.2009.10.026
Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, Mahadevan D (2015) First-in-human phase i dose escalation study of a second-generation non-ansamycin HSP90 Inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res 21(1):87–97. doi:10.1158/1078-0432.CCR-14-0979
Curry J, Angove H, Fazal L, Graham B, Harada I, Lyons J, Reule M, Smyth T, Thompson N (2009) Significance of long term pharmacodynamic actions of the HSP90 inhibitor AT13387. Proceedings of the AACR 100th Annual Meeting in Experimental and Molecular Therapeutics, Denver, CO: Abstract number 1856
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89(15):1138–1147
Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G (2011) A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res 17(21):6831–6839. doi:10.1158/1078-0432.CCR-11-0821
Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM (2006) Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther 5(10):2512–2521. doi:10.1158/1535-7163.MCT-06-0334
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18(1):64–76. doi:10.1158/1078-0432.CCR-11-1000
McCollum AK, Teneyck CJ, Sauer BM, Toft DO, Erlichman C (2006) Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res 66(22):10967–10975. doi:10.1158/0008-5472.CAN-06-1629
Smyth T, Paraiso KH, Hearn K, Rodriguez-Lopez AM, Munck JM, Haarberg HE, Sondak VK, Thompson NT, Azab M, Lyons JF, Smalley KS, Wallis NG (2014) Inhibition of HSP90 by AT13387 delays the emergence of resistance to BRAF inhibitors and overcomes resistance to dual BRAF and MEK inhibition in melanoma models. Mol Cancer Ther 13(12):2793–2804. doi:10.1158/1535-7163.MCT-14-0452
Acknowledgments
The authors thank Dr. Andrea Regier Voth, Leidos Biomedical Research, Inc., for medical writing support in the preparation of this manuscript, and Ms. Tracy Webb, Leidos Biomedical Research, Inc., for pharmacokinetic technical assistance. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and Core grant #CA16672. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. John Lyons is an employee of Astex Pharmaceuticals; no potential conflicts of interest were disclosed by the other authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Do, K., Speranza, G., Chang, LC. et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest New Drugs 33, 921–930 (2015). https://doi.org/10.1007/s10637-015-0255-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0255-1