Summary
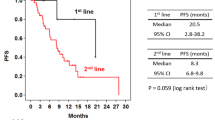
Background Preclinical and early clinical data support the use of Vascular Epithelial Growth Factor (VEGF)-targeted therapy with trastuzumab in Human Epidermal Receptor 2 (HER2) positive breast cancer. Adding bevacizumab to a taxane (docetaxel or paclitaxel) improves progression free survival (PFS) of metastatic breast cancer (MBC) patients. Objectives We evaluated the efficacy and feasibility of combining bevacizumab with trastuzumab and docetaxel in patients with HER2- positive MBC who received 0–1 prior chemotherapy regimens for metastatic disease. The primary end point was PFS. Materials and Methods Eligible patients received bevacizumab (15 mg/kg), trastuzumab (8 mg/kg loading dose followed by 6 mg/kg), and docetaxel (100 mg/m2 initially, later amended to 75 mg/m2) every three weeks for six cycles and then were allowed to receive bevacizumab and trastuzumab alone. Results Thirteen (50 %) of 26 patients enrolled completed all 6 cycles of bevacizumab, trastuzumab and docetaxel and went on to receive bevacizumab and trastuzumab alone (median: 11 cycles). The most common grade 3 or 4 toxicities include: neutropenia (8 %), septic death (4 %), infection not associated with neutropenia (15 %), fatigue (27 %), mylagia and/or arthraligia (20 %), and hand-foot syndrome (8 %). One patient (4 %) and six patients (23 %) developed grade 3 and grade 2 hypertension, respectively. Two (8 %) patients had transient grade 2 drop in Left Ventricular Ejection Fraction (LVEF) with full recovery later. The median progression free survival (PFS) was 14.3 months (95 % CI: 9.3–35 months), the objective response rate (ORR), defined as the best response of complete response (CR) or partial response (PR) was (12/26) 46 %. The clinical benefit rate (CBR), defined as the best response of CR or PR or stable disease (SD) for at least 24 weeks, was (18/26) 69 % (95 % CI: 48–86 %). Conclusion The combination of bevacizumab, trastuzumab and docetaxel is well tolerated and is clinically active in patients with HER2-positive MBC, with response rate and PFS comparable to previous reports utilizing higher dose of docetaxel (100 mg/m2). Recent randomized trials did not demonstrate additional overall survival (OS) benefit of adding bevacizumab to trastuzumab and docetaxel despite an improvement in PFS. Identification of predictive biomarkers and careful patient selection should be incorporated in further investigation of anti-VEGF in breast cancer.


Similar content being viewed by others
References
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. doi:10.1056/nejm200103153441101
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol Off J Am Soc Clin Oncol 23(19):4265–4274. doi:10.1200/jco.2005.04.173
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. doi:10.1056/NEJMoa052122
Dang C, Fornier M, Sugarman S, Troso-Sandoval T, Lake D, D’Andrea G, Seidman A, Sklarin N, Dickler M, Currie V, Gilewski T, Moynahan ME, Drullinsky P, Robson M, Wasserheit-Leiblich C, Mills N, Steingart R, Panageas K, Norton L, Hudis C (2008) The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(8):1216–1222. doi:10.1200/jco.2007.12.0733
Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, Meloche S, Alaoui-Jamali MA (2000) Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene 19(31):3460–3469. doi:10.1038/sj.onc.1203685
Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D (2002) ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer 94(11):2855–2861. doi:10.1002/cncr.10553
Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD (2004) Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res 10(5):1706–1716
Ramaswamy B, Elias AD, Kelbick NT, Dodley A, Morrow M, Hauger M, Allen J, Rhoades C, Kendra K, Chen HX, Eckhardt SG, Shapiro CL (2006) Phase II trial of bevacizumab in combination with weekly docetaxel in metastatic breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res 12(10):3124–3129. doi:10.1158/1078-0432.ccr-05-2603
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676. doi:10.1056/NEJMoa072113
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(20):3239–3247. doi:10.1200/jco.2008.21.6457
Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL (2009) Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 27(30):4966–4972. doi:10.1200/jco.2008.21.6630
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 29(10):1252–1260. doi:10.1200/jco.2010.28.0982
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791. doi:10.1056/NEJMoa040766
Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N, Goldhirsch A, Sandri MT (2008) Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 19(5):891–897. doi:10.1093/annonc/mdm558
Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K, Suzuki E (2010) Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast cancer (Tokyo, Japan) 17(3):199–204. doi:10.1007/s12282-009-0139-3
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res: Off J Am Assoc Cancer Res 10(20):6897–6904. doi:10.1158/1078-0432.ccr-04-0378
Maestro LM, Sastre J, Rafael SB, Veganzones SB, Vidaurreta M, Martin M, Olivier C, VB DELO, Garcia-Saenz JA, Alfonso R, Arroyo M, Diaz-Rubio E (2009) Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res 29(11):4839–4843
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, Isaacs C (2009) Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 27(31):5153–5159. doi:10.1200/jco.2008.20.6664
Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V (2013) AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 31(14):1719–1725. doi:10.1200/jco.2012.44.7912
Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De Laurentiis M, De Placido S, Valero V, Hortobagyi GN, Reuben JM, Cristofanilli M (2011) Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast cancer research : BCR 13(3):R67. doi:10.1186/bcr2907
Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Dieras V, Rolland E, Mignot L, Mathiot C, Bidard FC (2012) High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 23(3):618–624. doi:10.1093/annonc/mdr263
Schwartzberg LS, Badarinath S, Keaton MR, Childs BH (2013) Phase II multicenter study of docetaxel and Bevacizumab with or without Trastuzumab as first-line treatment for patients with metastatic breast cancer. Clinical breast cancer. doi:10.1016/j.clbc.2013.12.003
Martin M, Makhson A, Gligorov J, Lichinitser M, Lluch A, Semiglazov V, Scotto N, Mitchell L, Tjulandin S (2012) Phase II study of bevacizumab in combination with trastuzumab and capecitabine as first-line treatment for HER-2-positive locally recurrent or metastatic breast cancer. Oncologist 17(4):469–475. doi:10.1634/theoncologist.2011-0344
Lin NU, Seah DS, Gelman R, Desantis S, Mayer EL, Isakoff S, Dipiro P, Krop IE, Come SE, Weckstein D, Winer EP, Burstein HJ (2013) A phase II study of bevacizumab in combination with vinorelbine and trastuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res Treat 139(2):403–410. doi:10.1007/s10549-013-2551-9
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 23(15):3502–3508. doi:10.1200/jco.2005.10.017
Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R (2013) Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 14(10):933–942. doi:10.1016/s1470-2045(13)70335-8
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol Off J Am Soc Clin Oncol 26(28):4672–4678. doi:10.1200/jco.2008.16.1612
Etienne-Grimaldi MC, Formento P, Degeorges A, Pierga JY, Delva R, Pivot X, Dalenc F, Espie M, Veyret C, Formento JL, Francoual M, Piutti M, de Cremoux P, Milano G (2011) Prospective analysis of the impact of VEGF-A gene polymorphisms on the pharmacodynamics of bevacizumab-based therapy in metastatic breast cancer patients. Br J Clin Pharmacol 71(6):921–928. doi:10.1111/j.1365-2125.2010.03896.x
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119. doi:10.1056/NEJMoa1113216
Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ (2013) Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol Off J Am Soc Clin Oncol 31(9):1219–1230. doi:10.1200/jco.2012.46.2762
Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, Tomczak P, Provencher L, Cortes J, Delmar PR, Scherer SJ (2013) Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 108(5):1052–1060. doi:10.1038/bjc.2013.69
Munzone E, Botteri E, Sandri MT, Esposito A, Adamoli L, Zorzino L, Sciandivasci A, Cassatella MC, Rotmensz N, Aurilio G, Curigliano G, Goldhirsch A, Nole F (2012) Prognostic value of circulating tumor cells according to immunohistochemically defined molecular subtypes in advanced breast cancer. Clinical breast cancer 12(5):340–346. doi:10.1016/j.clbc.2012.07.001
Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabieres C, Janni W, Messina C, Paoletti C, Muller V, Hayes DF, Piccart M, Pierga JY (2013) Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev 32(1–2):179–188. doi:10.1007/s10555-012-9398-0
Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, Ueno NT, Andreopoulou E, Alvarez RH, Valero V, De Placido S, Hortobagyi GN, Reuben JM, Cristofanilli M (2012) Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 23(5):1144–1150. doi:10.1093/annonc/mdr434
Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, Veyret C, Bergougnoux L, de Cremoux P, Milano G, Pierga JY (2010) Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 21(9):1765–1771. doi:10.1093/annonc/mdq052
Wallwiener M, Hartkopf AD, Baccelli I, Riethdorf S, Schott S, Pantel K, Marme F, Sohn C, Trumpp A, Rack B, Aktas B, Solomayer EF, Muller V, Janni W, Schneeweiss A, Fehm TN (2013) The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat 137(2):503–510. doi:10.1007/s10549-012-2382-0
Arteaga CL, Mayer IA, O’Neill AM, Swaby RF, Perez EA, Lin NU, Sledge GW (2012) A randomized phase III double-blinded placebo-controlled trial of first-line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-overexpressing metastatic breast cancer (HER2 positive MBC): A trial of the Eastern Cooperative Oncology Group (E1105). 30:suppl; abstr 605
Slamon DJ, Swain SM, Buyse M, Martin M, Geyer CE, Im Y-H, Pienkowski T, Kim S-B, Robert NJ, Steger G, Crown J, Verma S, Eiermann W, JP C, Im S-A, Mamouna sE, Schwartzberg L, Paterson A, JR M, L P, MF P, M T, Bee-Munteanu V, Henschel V, Crepelle-Flechais A, Wolmark N (2013) Primary results from BETH, a phase 3 controlled study of adjuvant chemotherapy and trastuzumab ± bevacizumab in patients with HER2-positive, node-positive or high risk node-negative breast cancer. 36th Annual San Antonio Breast Cancer Symposium (SABCS)/AACR American Association for Cancer Research:S1-03
Yardley DA, Raefsky E, Castillo R, Lahiry A, Locicero R, Thompson D, Shastry M, Burris HA 3rd, Hainsworth JD (2011) Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clinical breast cancer 11(5):297–305. doi:10.1016/j.clbc.2011.04.002
Pierga JY, Petit T, Delozier T, Ferrero JM, Campone M, Gligorov J, Lerebours F, Roche H, Bachelot T, Charafe-Jauffret E, Pavlyuk M, Kraemer S, Bidard FC, Viens P (2012) Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 13(4):375–384. doi:10.1016/s1470-2045(12)70049-9
Conflict of interest disclosure
Dr. Bhuvaneswari Ramaswamy had disclosed that she had served on the advisory board for Genentech.
Ethical approval
All patients signed an informed consent form prior to enrollment in the study. Local ethical approval was received and the study was conducted in accordance with the Ohio State University and University of Pittsburgh Institutional Review Boards.
Funding
This work was supported by Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, M., Pan, X., Layman, R. et al. A Phase II study of bevacizumab in combination with trastuzumab and docetaxel in HER2 positive metastatic breast cancer. Invest New Drugs 32, 1285–1294 (2014). https://doi.org/10.1007/s10637-014-0122-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0122-5