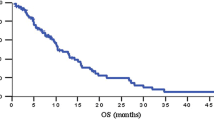
Summary
Background A Phase I trial of the 2-drug regimen of everolimus plus gemcitabine (Cohort I) and the 3-drug regimen of everolimus plus gemcitabine and cisplatin (Cohort II) was performed to determine the maximally tolerated dose (MTD) of both combinations. An expansion cohort (Cohort III) of patients with cholangiocarcinoma or gallbladder carcinoma was treated at the MTD. Methods A standard 3 + 3 design dose escalation was used. Everolimus was given on Monday/Wednesday/Friday or daily depending upon the dose level. Gemcitabine and cisplatin were administered on days 1 and 8 of each 21 day cycle. Results Twelve patients were entered in Cohort I and 15 in Cohort II. The MTD for Cohort I was everolimus 5 mg on Monday/Wednesday/Friday and gemcitabine 800 mg/m2. For Cohort II, it was everolimus 5 mg on Monday/Wednesday/Friday, gemcitabine 600 mg/m2, and cisplatin 12.5 mg/m2. All DLTs in this study were hematologic. Complete responses (CR) were seen in a patient with primary peritoneal carcinoma and another with recurrent pancreatic cancer. Partial responses (PR) were seen in 3 patients: breast, ampullary carcinoma and pheochromocytoma. Of 10 patients enrolled in Cohort III, six patients had stable disease and 4 had progressive disease. Conclusions This Phase I clinical trial has demonstrated that these 2-drug and 3-drug combinations are generally well tolerated and safely administered. The main DLTs in both regimens were hematologic, specifically thrombocytopenia. The 3-drug combination can be considered as a platform for future studies in specific tumor types.
Similar content being viewed by others
References
Novartis. RAD001 Investigators Brochure (2011)
O’Donnell A, Faivre S, Burris HA et al (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588–95
Tanaka C, O’Reilly T, Kovarik JM et al (2008) Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 26:1596–602
O’Reilly T, McSheehy PM, Wartmann M, Lassota P, Brandt R, Lane HA (2011) Evaluation of the mTOR inhibitor, everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anticancer Drugs 22:58–78
Castro MP (1998) Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer 82:639–41
Gallardo J, Fodor M, Gamargo C, Orlandi L (1998) Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer 83:2419–21
Kovarik JM, Sabia HD, Figueiredo J et al (2001) Influence of hepatic impairment on everolimus pharmacokinetics: implications for dose adjustment. Clin Pharmacol Ther 70:425–30
Peveling-Oberhag J, Zeuzem S, Yong WP et al (2013) Effects of hepatic impairment on the pharmacokinetics of everolimus: a single-dose, open-label parallel-group study. Clin Ther 35:215–25
Ito D, Fujimoto K, Mori T et al (2006) In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer 118:2337–43
Pacey S, Rea D, Steven N et al (2004) Results of a phase 1 clinical trial investigating a combination of the oral mTOR-inhibitor Everolimus (E, RAD001) and Gemcitabine (GEM) in patients (pts) with advanced cancers. J Clin Oncol 22:3120
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–81
Bukowski RM, Leichman LP, Rivkin SE (1983) Phase II trial of m-AMSA in gallbladder and cholangiocarcinoma: a Southwest oncology group study. Eur J Cancer Clin Oncol 19:721–3
Ekstrom K, Hoffman K, Linne T, Eriksson B, Glimelius B (1998) Single-dose etoposide in advanced pancreatic and biliary cancer, a phase II study. Oncol Rep 5:931–4
Jones DV Jr, Lozano R, Hoque A, Markowitz A, Patt YZ (1996) Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol 14:2306–10
Okada S, Ishii H, Nose H et al (1994) A phase II study of cisplatin in patients with biliary tract carcinoma. Oncol 51:515–7
Taal BG, Audisio RA, Bleiberg H et al (1993) Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol 4:607–9
Fury MG, Sherman E, Haque S, et al. (2011). A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol
Conflict of Interest
Dr. Mitesh Borad has received grant funding from Novartis Pharmaceuticals for unrelated research. All other authors declare they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by Novartis Pharmaceuticals and in part by Mayo Clinic Cancer Center (CA15083)
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Rights and permissions
About this article
Cite this article
Costello, B.A., Borad, M.J., Qi, Y. et al. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Invest New Drugs 32, 710–716 (2014). https://doi.org/10.1007/s10637-014-0096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0096-3