Summary
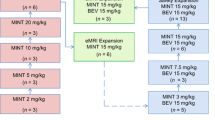
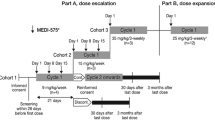
The human monoclonal antibody MNRP1685A targets the VEGF binding domain of neuropilin-1 (NRP1), a multi-domain receptor necessary for neural development and blood vessel maturation. In nonclinical studies, MNRP1685A prevents vascular maturation by keeping blood vessels in an immature, highly VEGF-dependent state. We explored the safety and tolerability of MNRP1685A in patients with advanced solid tumors. Patients were treated with MNRP1685A given intravenously every 3 weeks using a 3 + 3 dose-escalation design with 7 dose-escalation cohorts. Twenty-four of 35 patients (69 %) experienced drug-related adverse events (AEs) of infusion-related reaction on the day of MNRP1685A administration. With premedication including dexamethasone, infusions were well-tolerated with main symptoms of pruritus and rash. Outside the day of infusion, most common (≥ 2 patients) related AEs were fatigue (17 %), pruritus (9 %), myalgia and thrombocytopenia (both 6 %) (all were Grade 1–2). MNRP1685A-related Grade ≥ 3 AEs consisted of one dose-limiting toxicity of Grade 3 upper gastrointestinal bleeding and one related Grade 3 thrombocytopenia, coinciding with unrelated Grade 3 fungemia and duodenal obstruction. MNRP1685A showed nonlinear PK with more-than-dose proportional increases in exposure, consistent with broad target expression. Transient platelet count reductions (≥ 30 % from predose) were observed in 56 % of evaluable patients. Nine patients were on study for ≥ 4 cycles, one colorectal cancer patient for one year. MNRP1685A was generally well-tolerated. The primary MNRP1685A-related AE was infusion-related reaction, which were attenuated by premedication including dexamethasone. Transient platelet count reductions were frequent but did not impact MNRP1685A dosing.



Similar content being viewed by others
References
Tam SJ, Watts RJ (2010) Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci 33:379–408
He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90:739–51
Soker S, Takashima S, Quan H et al (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–45
Kawasaki T, Kitsukawa T, Bekku Y (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895–902
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M (2002) Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277:24818–25
Takashima S, Kitakaze M, Asakura M et al (2002) Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A 99:3657–62
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C (2004) Neuropilin-1is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231:503–9
Grandclement C, Borg C (2011) Neuropilins: a new target for cancer therapy. Cancers 3:1899–1928
Bielenberg DR, Pettaway CA, Takashima S et al (2006) Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res 312:584–93
Beck B, Driessens G, Goossens S et al (2011) A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumors. Nature 478:399–403
Xin Y, Bai S, Damico-Beyer LA et al (2012) Anti-neuropilin-1 (MRNP1685A): unexpected pharmacokinetic differences across species, from preclinical models to humans. Pharm Res 29:2512–21
Xin Y, Li J, Wu J, et al. (2012b) Pharmacokinetic and pharmacodynamic analysis of circulating biomarkers of anti-NRP1, a novel anti-angiogenesis agent, in two Phase I trials in patients with advanced solid tumors, Clinical Cancer Research, in press.
Liang W-C, Dennis MS, Stawicki S et al (2007) Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol 366:815–29
Pan Q, Chanthery Y, Liang W-C (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11:53–67
Bugelski PJ, Achuthanandam R, Capocasale RJ et al (2009) Monoclonal antibody-induced cytokine-release syndrome. Expert Rev Clin Immunol 5:499–521
Xia CQ, Peng R, Beato F, Clare-Salzler MJ (2005) Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand J Immunol 62:45–54
Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A (2012) IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction–a comparative study of human clinical-applicable DC. Clin Immunol 142:332–42
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–41
Darbonne WC, Du X, Dhawan P, et al (2011) Mechanism for platelet reduction in anti-neuropilin-1 (MNRP1685A)-treated phase I patients. J Clin Oncol 29
Grothey A, Galanis E (2009) Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol 6:507–18
Raja FA, Chopra N, Ledermann JA (2010) Optimal first-line treatment in ovarian cancer. Ann Oncol 23(10):x118–x127
Pellet-Many C, Frankel P, Jia H (2008) Neuropilins: structure, function and role in disease. Biochem J 411:211–26
Pataik A, LoRusso PM, Messersmith W (2013) A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. (in press)
Acknowledgement
We thank the patients who participated in the study and their families. Medical writing assistance provided by Genentech, Inc.
Research support
This study was sponsored by Genentech, Inc.
Disclosure information
CDW: advisor to and research support from Genentech, Inc.
MB: research support from Genentech, Inc.
AWT: advisor to and research support from Genentech, Inc.
KP: nothing to disclose
LG: nothing to disclose
PH, YX, RY, LMS, HX, and RKB: employees of Genentech, Inc., and shareholders of F. Hoffmann La Roche, Ltd.
AP: advisor to and research support from Genentech, Inc.
Author contributions
CDW: conception and design, collection and assembly of data, data analysis and interpretation, provision of study patients
MB: collection and assembly of data
AWT: collection and assembly of data, provision of study patients
KP: collection and assembly of data
LG: data analysis and provision of study patients
PH: conception and design, data analysis and interpretation
YX: data analysis and interpretation
RY: data analysis and interpretation
LMS: data analysis and interpretation
HX: conception and design, data analysis and interpretation
RKB: conception and design, collection and assembly of data, data analysis and interpretation
AP: conception and design, collection and assembly of data, data analysis and interpretation, provision of study patients
All authors participated in manuscript writing, and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(PDF 500 kb)
Supplementary Table 1
(DOC 38 kb)
Supplementary Table 2
(DOC 59 kb)
Supplemental Table 3
(DOC 34 kb)
Supplemental Table 4
(DOC 36 kb)
Rights and permissions
About this article
Cite this article
Weekes, C.D., Beeram, M., Tolcher, A.W. et al. A phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Invest New Drugs 32, 653–660 (2014). https://doi.org/10.1007/s10637-014-0071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0071-z