Summary
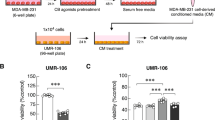
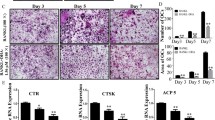
Breast cancer frequently spreads to bone. The interaction between bone metastases and microenvironment, referred as the “vicious cycle”, increases both tumor burden and bone destruction. Therefore, inhibition at any point in this “vicious cycle” can reduce malignant osteolytic lesions in patients with advanced breast cancer. In this study, we evaluated whether tetrahydrofurofuran-type lignans derived from Magnoliae Flos, commonly used in traditional Asian medicine to treat inflammatory diseases, could block breast cancer-mediated bone loss. Aschatin, fargesin, lirioresinol B dimethyl ether, and magnolin at noncytotoxic concentrations suppressed mRNA expression and secretion of osteolytic factor PTHrP in MDA-MB-231 metastatic human breast cancer cells. Fargesin inhibited TGF-β-stimulated cell viability, migration, and invasion and decreased TGF-β-induced PTHrP production in MDA-MB-231 cells. In addition, these lignans reduced RANKL/OPG ratio in PTHrP-treated hFOB1.19 human osteoblastic cells and inhibited RANKL-mediated osteoclast differentiation in mouse bone marrow macrophages. Aschatin, fargesin, lirioresinol B dimethyl ether, and magnolin substantially reduced bone-resorbing activity of osteoclasts by inhibiting MMP-9 and cathepsin K activities. Furthermore, orally administered fargesin inhibited tumor growth and cancer-mediated bone destruction in mice with MDA-MB-231 cells injected into calvarial tissues. Aschatin, fargesin, lirioresinol B dimethyl ether, and magnolin blocked initiation and progression of the “vicious cycle” between breast cancer metastases and bone microenvironment by inhibiting PTHrP production in breast cancer cells and osteoclastic bone resorption. Therefore, these tetrahydrofurofuran-type lignans have the potential to serve as beneficial agents to prevent and treat cancer-induced bone destruction in breast cancer patients.







Similar content being viewed by others
References
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396
Watts NB (1999) Clinical utility of biochemical markers of bone remodeling. Clin Chem 45:1359–1368
Clezardin P, Teti A (2007) Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis 24:599–608
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220
Kim EK, Song MY, Kim IS, Moon WS, Ryu DG, So HS, Park R, Park JW, Kwon KB, Park BH (2008) Beneficial effect of Flos magnoliae extract on multiple low dose streptozotocin-induced type 1 diabetes development and cytokine-induced beta-cell damage. Int J Mol Med 22:481–488
Shen Y, Li CG, Zhou SF, Pang EC, Story DF, Xue CC (2008) Chemistry and bioactivity in Flos Magnoliae, a Chinese herb for rhinitis and sinusitis. Curr Med Chem 15:1616–1627
Lee J, Lee D, Jang DS, Nam JW, Kim JP, Park KH, Yang MS, Seo EK (2007) Two new stereoisomers of Tetrahydrofuranoid lignans from the flower buds of Magnolia fargesii. Chem Pharm Bull (Tokyo) 55:137–139
Schühly W, Skarbina J, Kunert O, Nandi OI, Bauer R (2009) Chemical characterization of Magnolia biondii (Flos Magnoliae, Xin Yi). Nat Prod Commun 4:231–234
Lim H, Son KH, Bae KH, Hung TM, Kim YS, Kim HP (2009) 5-lipoxygenase-inhibitory constituents from Schizandra fructus and Magnolia flos. Phytother Res 23:1489–1492
Kim JS, Kim JY, Lee HJ, Lim HJ, Lee DY, Kim DH, Ryu JH (2010) Suppression of inducible nitric oxide synthase expression by furfuran lignans from flower buds of Magnolia fargesii in BV-2 microglial cells. Phytother Res 24:748–753
Jun AY, Kim HJ, Park KK, Son KH, Lee DH, Woo MH, Kim YS, Lee SK, Chung WY (2012) Extract of Magnoliae Flos inhibits ovariectomy-induced osteoporosis by blocking osteoclastogenesis and reducing osteoclast-mediated bone resorption. Fitoterapia 83:1523–1531
Guise TA (2009) Breaking down bone: new insight into site-specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Genes Dev 23:2117–2123
Yin JJ, Selander K, Chirgwin J, Dallas M, Grubbs B, Wieser R, Massagué J, Mundy GR, Guise TA (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103:197–206
Wittrant Y, Théoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D, Rédini F (2004) RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta 1704:49–57
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55:61–66
Zhang Y, Ma B, Fan Q (2010) Mechanisms of breast cancer bone metastasis. Cancer Lett 292:1–7
Sterling JA, Edwards JR, Martin TJ, Mundy GR (2011) Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone 48:6–15
Coleman RE, Seaman JJ (2001) The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. Semin Oncol 28:11–16
Kohno N (2008) Treatment of breast cancer with bone metastasis: bisphosphonate treatment - current and future. Int J Clin Oncol 13:18–23
Santini D, Galluzzo S, Zoccoli A, Pantano F, Fratto ME, Vincenzi B, Lombardi L, Gucciardino C, Silvestris N, Riva E, Rizzo S, Russo A, Maiello E, Colucci G, Tonini G (2010) New molecular targets in bone metastases. Cancer Treat Rev 36(Suppl 3):S6–S10
Zheng Y, Zhou H, Brennan K, Blair JM, Modzelewski JR, Seibel MJ, Dunstan CR (2007) Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone 40:471–478
Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM (2006) Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 12:6213s–6216s
Roodman GD (2004) Mechanisms of bone metastasis. N Eng J Med 350:1655–1664
Saito H, Tsunenari T, Onuma E, Sato K, Ogata E, Yamada-Okabe H (2005) Humanized monoclonal antibody against parathyroid hormone-related protein suppresses osteolytic bone metastasis of human breast cancer cells derived from MDA-MB-231. Anticancer Res 25:3817–3823
Soki FN, Park SI, McCauley LK (2012) The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol 8:803–817
Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA (2002) Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277:24571–24578
Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, Matrisian LM, Mundy GR, Sterling JA (2011) TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res 71:822–831
Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A (2011) TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res 71:5606–5610
McGrath EE (2011) OPG/RANKL/RANK pathway as a therapeutic target in cancer. J Thorac Oncol 6:1468–1473
Andersen TL, del Carmen Ovejero M, Kirkegaard T, Lenhard T, Foged NT, Delaissé JM (2004) A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone 35:1107–1119
Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP (2011) Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol 7:447–456
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010–0026040).
Declaration of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jun, A.Y., Kim, HJ., Park, KK. et al. Tetrahydrofurofuran-type lignans inhibit breast cancer-mediated bone destruction by blocking the vicious cycle between cancer cells, osteoblasts and osteoclasts. Invest New Drugs 32, 1–13 (2014). https://doi.org/10.1007/s10637-013-9969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9969-0