Summary
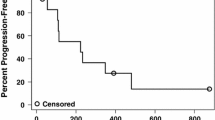
Background Preclinical data have shown that lenalidomide and sorafenib target endothelial cells, inhibiting growth of ocular melanoma cells in a xenograft model. We conducted a Phase I study of lenalidomide and sorafenib in patients with advanced cancer. Methods During the escalation phase, lenalidomide (days 1–21) and sorafenib (days 1–28) were given orally once daily at the following respective doses: level 1 (10 mg, 200 mg); level 2 (10 mg, 400 mg); level 3 (20 mg, 400 mg); and level 4 (25 mg, 400 mg) (1 cycle = 28 days). A “3 + 3” study design was used. Results Forty-one patients were treated (median age: 50 years). The most common diagnoses were adenoid cystic carcinoma (N = 9), ovarian adenocarcinoma (N = 7), and melanoma (N = 6); 142 cycles (median: 3) were administered. No dose-limiting toxicities were noted. The maximum tested dose (dose level 4) was used in the expansion phase. Grade 3–4 treatment-related toxicities were neutropenia, thrombocytopenia, skin rash, and thromboembolism. Of 38 patients who were evaluable for response, stable disease (SD) was noted in 53 % of patients (SD ≥6 months: 16 %). Tumor types with SD ≥ 6 months were as follows: ocular melanoma, 2/2 (100 %); other melanoma, 1/4 (25 %); adenoid cystic carcinoma, 2/9 (22 %); and ovarian cancer, 1/6 (17 %). The median progression-free survival duration was 3.5 months (95 % CI, 1.9–5.0), and the median overall survival duration was 12.3 months (95 % CI, 10.1–14.5). Conclusions Lenalidomide and sorafenib was well tolerated and associated with disease stabilization for ≥6 months in patients with melanoma, adenoid cystic carcinoma, and ovarian adenocarcinoma.


Similar content being viewed by others
References
Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB (2005) Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res 69(1–2):56–63
Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163(1):380–386
Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, Wolbring G, Govinda S, Corral LG, Payvandi F, Muller GW, Stirling DI (2003) Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther 305(3):1222–1232
Segler A, Tsimberidou AM (2012) Lenalidomide in solid tumors. Cancer Chemother Pharmacol 69(6):1393–1406
Petrylak D, Resto-Garces K, Tibyan M, Mohile S (2009) A phase I open-label study using lenalidomide and docetaxel in castration- resistant prostate cancer. J Clin Oncol 7(suppl), abstr 5156
Papadopoulos K, Mendelson D, Preston G (2005) A phase I study of lenalidomide and weekly docetaxel in patients with advanced solid tumors. Clin Cancer Res: Abstr Int Conf Mol Targets Cancer Ther 11(24 Pt 2):215
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5(10):835–844
Mangiameli DP, Blansfield JA, Kachala S, Lorang D, Schafer PH, Muller GW, Stirling DI, Libutti SK (2007) Combination therapy targeting the tumor microenvironment is effective in a model of human ocular melanoma. J Transl Med 5:38
Institute NC (2006) Common Terminology Criteria for Adverse Events v3.0 (CTCAE). National Cancer Institute. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed December 18 2012
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O’Dwyer PJ (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512
Sharma RA, Steward WP, Daines CA, Knight RD, O’Byrne KJ, Dalgleish AG (2006) Toxicity profile of the immunomodulatory thalidomide analogue, lenalidomide: phase I clinical trial of three dosing schedules in patients with solid malignancies. Eur J Cancer 42(14):2318–2325
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B (2007) Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12(4):426–437
Eisen T, Trefzer U, Hamilton A, Hersey P, Millward M, Knight RD, Jungnelius JU, Glaspy J (2010) Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer 116(1):146–154
Glaspy J, Atkins MB, Richards JM, Agarwala SS, O’Day S, Knight RD, Jungnelius JU, Bedikian AY (2009) Results of a multicenter, randomized, double-blind, dose-evaluating phase 2/3 study of lenalidomide in the treatment of metastatic malignant melanoma. Cancer 115(22):5228–5236
Amato RJ, Hernandez-McClain J, Saxena S, Khan M (2008) Lenalidomide therapy for metastatic renal cell carcinoma. Am J Clin Oncol 31(3):244–249
Choueiri TK, Dreicer R, Rini BI, Elson P, Garcia JA, Thakkar SG, Baz RC, Mekhail TM, Jinks HA, Bukowski RM (2006) Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer 107(11):2609–2616
Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C, Luppi G, Conte P (2010) Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 102(1):68–72
Ott PA, Hamilton A, Min C, Safarzadeh-Amiri S, Goldberg L, Yoon J, Yee H, Buckley M, Christos PJ, Wright JJ, Polsky D, Osman I, Liebes L, Pavlick AC (2010) A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One 5(12):e15588
Figueiras RG, Padhani AR, Goh VJ, Vilanova JC, Gonzalez SB, Martin CV, Caamano AG, Naveira AB, Choyke PL (2011) Novel oncologic drugs: what they do and how they affect images. Radiographics 31(7):2059–2091
Garrido-Laguna I, Janku F, Vaklavas C, Falchook GS, Fu S, Hong DS, Naing A, Tsimberidou AM, Wen S, Kurzrock R (2012) Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer 118(5):1422–1428
Funding source
This research is supported in part by Celgene (provided free lenalidomide and a research grant to Dr. Tsimberidou).
Conflicts of interest
P. Ganesan: None. S. Piha-Paul: None. A. Naing: None. J. Wheler: None. S. Fu: None. D.S. Hong: None. R. Kurzrock: None. F. Janku: None. S. Laday: None. A.Y. Bedikian: None. M. Kies: None. R. Wolff: None. A.M. Tsimberidou and G. Falchook: Received research funding from Celgene.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganesan, P., Piha-Paul, S., Naing, A. et al. Phase I clinical trial of lenalidomide in combination with sorafenib in patients with advanced cancer. Invest New Drugs 32, 279–286 (2014). https://doi.org/10.1007/s10637-013-9966-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9966-3