Summary
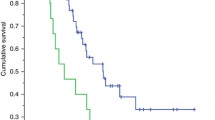
Background Low muscle mass has been associated with chemotherapy toxicity. We conducted this prospective study to evaluate the effects of body composition on the occurrence of toxicity in phase I trials. Patients and Methods Patients were consecutively enrolled irrespective of the type of tumor or the type of drug. The Skeletal Muscle Index (SMIndex) and visceral and subcutaneous adipose tissue were assessed with computed tomography imaging by measuring cross-sectional areas of the tissues (cm2/m2). Dose-limiting toxicity (DLT) corresponded to toxicities occurring during the 1st cycle that necessitated dose reduction, postponement or interruption of drug administration and severe toxicity events (STE) corresponded to DLT or permanent treatment withdrawal due to toxicity. Results 93 patients were evaluated. Ten percent of patients experienced DLT and had a lower SMIndex: 40.8 ± 4.6 vs. 48.1 ± 9.6 cm2/m2 (p = 0.01). STE occurred in 14 % of the patients. The only factor associated with STE was a low SMIndex: 42.4 ± 5.8 vs. 48.4 ± 9.7 cm2/m2 (p = 0.02). STE were observed in 25.5 % of the patients when the SMIndex was below the median value compared to 6.5 % of patients with a high SMIndex (p = 0.02). Conclusion Muscle mass is a critical predictor of severe toxicity events in phase I patients, suggesting that sarcopenia may be considered in assessing patients for eligibility of phase-1 studies.


Similar content being viewed by others
References
Italiano A, Massard C, Bahleda R et al (2008) Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol 19:787–792
Wheler J, Tsimberidou AM, Hong D et al (2012) Survival of 1181 patients in a phase I clinic: the MD anderson clinical center for targeted therapy experience. Clin Cancer Res 18:2922–2929
Olmos D, A’hern RP, Marsoni S et al (2012) Patient selection for oncology phase I trials: a multi-institutional study of prognostic factors. J Clin Oncol 30:996–1004
LoConte NK, Smith M, Alberti D et al (2010) Amongst eligible patients, age and comorbidity do not predict for dose-limiting toxicity from phase I chemotherapy. Cancer Chemother Pharmacol 65:775–780
Meyerhardt JA, Tepper JE, Niedzwiecki D et al (2004) Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol 22:648–657
Telli L, Witteles RM, Fisher GA, Srinivas S (2008) Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 19:1613–1618
Mitsiopoulos N, Baumgartner RN, Heymsfield SB et al (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Mourtzakis M, Prado CMM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Prado CMM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Tan BHL, Birdsell LA, Martin L et al (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15:6973–6979
Prado CMM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Prado CMM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Antoun S, Baracos VE, Birdsell L et al (2010) Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 21:1594–1598
Mir O, Coriat R, Blanchet B et al (2012) Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. Plos One 7:e37563
Huillard O, Mir O, Peyromaure M et al (2013) Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108:1034–1041
Massicotte MH, Borget I, Broutin S et al (2013) Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: Results from a placebo-controlled study. J Clin Endocrinol Metab 98:2401–2408
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Gusella M, Toso S, Ferrazzi E et al (2002) Relationships between body composition parameters and fluorouracil pharmacokinetics. Br J Clin Pharmacol 54:131–139
Nawaratne S, Brien JE, Seeman E et al (1998) Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol 46:447–452
Morgan DJ, Bray KM (1994) Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet 26:292–307
Khamoui AV, Kim JS (2012) Candidate mechanisms underlying effects of contractile activity on muscle morphology and energetics in cancer cachexia. Eur J Cancer Care 21:143–157
Acknowledgments
The authors express their gratitude to Lorna Saint Ange for editing.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Disclosure
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cousin, S., Hollebecque, A., Koscielny, S. et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs 32, 382–387 (2014). https://doi.org/10.1007/s10637-013-0053-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0053-6