Summary
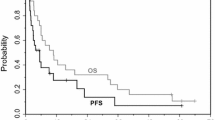
Purpose Preclinical data showed that trientine, a copper-lowering agent, re-sensitized cancer cells to carboplatin through enhanced human copper transporter 1 (hCtr1) -mediated platinum uptake. Experimental Design We studied carboplatin and trientine in patients (n = 55; 45 who had failed platinum) with advanced malignancies (Phase I, modified 3 + 3 design). Results The most common cancers were head and neck (n = 13), non-small cell lung (n = 10) and epithelial ovarian (n = 8). The median number of prior regimens was four. No dose-limiting toxicity or treatment-related deaths were observed at doses up to carboplatin AUC 6 given with trientine. Eight patients achieved stable disease (SD) ≥ 6 months (six platinum failures) and one patient with platinum-resistant ovarian cancer, partial response (PR) (total SD ≥ 6 months/PR = 9, 16.4 %). The mean nadir serum copper level in the nine patients with SD ≥ 6 months/PR was 0.55 μg/mL (95 % CI, 0.34–0.75) versus 1.22 μg/mL (95 % CI, 1.02–1.42) (p < 0.001) in 38 tested patients with progression. In patients who maintained their ceruloplasmin (major copper-carrying protein) levels at 5–15 mg/dL (n = 9), the median progression-free and overall survivals were 9.2 and 15.2 months versus 1.9 (p = 0.001) and 5.7 months (p = 0.033) in patients who did not (n = 38), respectively. Conclusions The combination of a copper-lowering agent with carboplatin was well tolerated and associated with antitumor activity, especially in patients in whom copper and/or ceruloplasmin levels were lowered. Further investigation of this strategy for reversing platinum resistance is warranted.



Similar content being viewed by others
References
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nature reviews Drug discovery 4(4):307–320. doi:10.1038/nrd1691
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7(8):573–584. doi:10.1038/nrc2167
Shahzad MM, Lopez-Berestein G, Sood AK (2009) Novel strategies for reversing platinum resistance. Drug Resist Updat 12(6):148–152. doi:10.1016/j.drup.2009.09.001
Matsuo K, Lin YG, Roman LD, Sood AK (2010) Overcoming platinum resistance in ovarian carcinoma. Expert opinion on investigational drugs 19(11):1339–1354. doi:10.1517/13543784.2010.515585
Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, Sood AK, Wolf JK, Gershenson DM, Markman M, Hennessy BT, Kurzrock R, Bast RC Jr (2011) Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer 117(8):1661–1669. doi:10.1002/cncr.25701
Ishida S, Lee J, Thiele DJ, Herskowitz I (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proceedings of the National Academy of Sciences of the United States of America 99(22):14298–14302. doi:10.1073/pnas.162491399
Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Molecular cancer therapeutics 3(12):1543–1549
Liang ZD, Stockton D, Savaraj N, Tien Kuo M (2009) Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Molecular pharmacology 76(4):843–853. doi:10.1124/mol.109.056416
Sinani D, Adle DJ, Kim H, Lee J (2007) Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem 282(37):26775–26785. doi:10.1074/jbc.M703973200
Ishida S, McCormick F, Smith-McCune K, Hanahan D (2010) Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer cell 17(6):574–583. doi:10.1016/j.ccr.2010.04.011
Liang ZD, Long Y, Tsai WB, Fu S, Kurzrock R, Gagea-Iurascu M, Zhang F, Chen HH, Hennessy BT, Mills GB, Savaraj N, Kuo MT (2012) Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol Cancer Ther 11(11):2483–2494. doi:10.1158/1535-7163.MCT-12-0580
Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, Liu HS, Su WC (2012) Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer 75(2):228–234. doi:10.1016/j.lungcan.2011.06.011
Kim BE, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4(3) (3):176–185. doi: 10.1038/nchembio.72
Howell SB, Safaei R, Larson CA, Sailor MJ (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Molecular pharmacology 77(6):887–894. doi:10.1124/mol.109.063172
Liang ZD, Tsai WB, Lee MY, Savaraj N, Kuo MT (2012) Specificity protein 1 (sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol Pharmacol 81(3):455–464. doi:10.1124/mol.111.076422
Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T (2007) The roles of copper transporters in cisplatin resistance. Cancer metastasis reviews 26(1):71–83. doi:10.1007/s10555-007-9045-3
Lu J (2010) Triethylenetetramine pharmacology and its clinical applications. Molecular cancer therapeutics 9(9):2458–2467. doi:10.1158/1535-7163.MCT-10-0523
Kuo MT, Fu S, Savaraj N, Chen HH (2012) Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res 72(18):4616–4621. doi:10.1158/0008-5472.CAN-12-0888
ClinicalTrials.gov Available at: http://clinicaltrials.gov/ct2/show/NCT01178112. Accessed March 24, 2012
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25(13):1753–1759. doi:10.1200/JCO.2006.07.3049
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Available at: http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf. Accessed August 8, 2013
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Chan A, Wong F, Arumanayagam M (1993) Serum ultrafiltrable copper, total copper and caeruloplasmin concentrations in gynaecological carcinomas. Annals of clinical biochemistry 30(Pt 6):545–549
Muggia F (2009) Platinum compounds 30 years after the introduction of cisplatin: implications for the treatment of ovarian cancer. Gynecol Oncol 112(1):275–281. doi:10.1016/j.ygyno.2008.09.034
Shen DW, Pouliot LM, Hall MD, Gottesman MM (2012) Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 64(3):706–721. doi:10.1124/pr.111.005637
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22(47):7265–7279. doi:10.1038/sj.onc.1206933
Fu S, Naing A, Fu C, Kuo MT, Kurzrock R (2012) Overcoming platinum resistance through the use of a copper-lowering agent. Mol Cancer Ther 11(6):1221–1225. doi:10.1158/1535-7163.MCT-11-0864
Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, Lu J, Wu LY, Siddik ZH, Klomp LW, Savaraj N, Kuo MT (2008) Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Molecular pharmacology 74(3):697–704. doi:10.1124/mol.108.047969
Hayashi M, Nishiya H, Chiba T, Endoh D, Kon Y, Okui T (2007) Trientine, a copper-chelating agent, induced apoptosis in murine fibrosarcoma cells in vivo and in vitro. J Vet Med Sci 69(2):137–142
Yoshiji H, Yoshii J, Kuriyama S, Ikenaka Y, Noguchi R, Yanase K, Namisaki T, Kitade M, Yamazaki M, Fukui H (2005) Combination of copper-chelating agent, trientine, and methotrexate attenuates colorectal carcinoma development and angiogenesis in mice. Oncol Rep 14(1):213–218
Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, Tsujinoue H, Nakatani T, Kishida H, Nakae D, Gomez DE, De Lorenzo MS, Tejera AM, Fukui H (2001) The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int J Cancer 94(6):768–773. doi:10.1002/ijc.1537
Kavanagh J, Tresukosol D, Edwards C, Freedman R, Gonzalez de Leon C, Fishman A, Mante R, Hord M, Kudelka A (1995) Carboplatin reinduction after taxane in patients with platinum-refractory epithelial ovarian cancer. J Clin Oncol 13(7):1584–1588
Leitao MM Jr, Hummer A, Dizon DS, Aghajanian C, Hensley M, Sabbatini P, Venkatraman E, Spriggs DR (2003) Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol Oncol 91(1):123–129
Gartner EM, Griffith KA, Pan Q, Brewer GJ, Henja GF, Merajver SD, Zalupski MM (2009) A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Investigational new drugs 27(2):159–165. doi:10.1007/s10637-008-9165-9
Brewer GJ, Askari F, Dick RB, Sitterly J, Fink JK, Carlson M, Kluin KJ, Lorincz MT (2009) Treatment of Wilson’s disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res 154(2):70–77. doi:10.1016/j.trsl.2009.05.002
Acknowledgments
The authors thank Thuan Nguyen and Jing Gong in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for coordinating this clinical trial, and Diane Hackett and Jill Delsigne in the Department of Scientific Publications at MD Anderson for editing the manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, S., Hou, MM., Wheler, J. et al. Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Invest New Drugs 32, 465–472 (2014). https://doi.org/10.1007/s10637-013-0051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0051-8