Summary
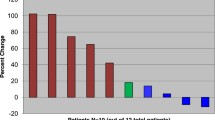
Ewing sarcoma is a rare connective tissue tumor characterized by the translocation of the EWS gene, mainly between chromosome 11 and 22, giving rise to gene re-arrangements between the EWS gene and various members of the ETS gene family. Multi-agent chemotherapy has improved the outcome for patients with localized Ewing sarcoma, but survival of patients with recurrent/metastatic disease remains poor. An exploratory two-stage, single-arm Phase II multicenter trial of the synthetic alkaloid, PM00104, was conducted in patients with recurrent Ewing sarcoma. The primary end point of the trial was objective response rate. PM00104 was administered at a dose of 2 mg/m2 on Days 1, 8 and 15 of a 4 week cycle. Seventeen patients were recruited. No objective responses were reported in the 16 patients evaluable for efficacy. Recruitment was closed without proceeding to the second stage of the trial. Four patients achieved stable disease as best response, and in two of these patients the stabilization was longer than 4 months. The median progression-free survival was 1.8 months (95 % CI, 0.9–3.5 months) and median overall survival was not reached (95%CI, 56.2 % at censored data). Pharmacokinetics in patients with Ewing sarcoma was similar to that previously reported in other patient populations. PM00104 showed modest activity in Ewing sarcoma at 2 mg/m2 on a weekly schedule. There remains an unmet need for effective therapies for patients with advanced/metastatic Ewing sarcoma.


Similar content being viewed by others
References
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G et al (1992) Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359(6391):162–165. doi:10.1038/359162a0
Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT (1994) A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet 6(2):146–151. doi:10.1038/ng0294-146
Shing DC, McMullan DJ, Roberts P, Smith K, Chin SF, Nicholson J, Tillman RM, Ramani P, Cullinane C, Coleman N (2003) FUS/ERG gene fusions in Ewing’s tumors. Cancer Res 63(15):4568–4576
Owens C, Abbott LS, Gupta AA (2013) Optimal management of ewing sarcoma family of tumors: recent developments in systemic therapy. Pediatr Drugs. doi:10.1007/s40272-013-0037-1
Leavey PJ, Collier AB (2008) Ewing sarcoma: prognostic criteria, outcomes and future treatment. Expert Rev Anticancer Ther 8(4):617–624. doi:10.1586/14737140.8.4.617
Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Brown K, Tarbell N, Bernstein ML, Granowetter L, Gebhardt M, Grier HE (2008) Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer 51(3):334–338. doi:10.1002/pbc.21618
Ocio EM, Maiso P, Chen X, Garayoa M, Alvarez-Fernandez S, San-Segundo L, Vilanova D, Lopez-Corral L, Montero JC, Hernandez-Iglesias T, de Alava E, Galmarini C, Aviles P, Cuevas C, San-Miguel JF, Pandiella A (2009) Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood 113(16):3781–3791. doi:10.1182/blood-2008-09-177774
Leal JF, Garcia-Hernandez V, Moneo V, Domingo A, Bueren-Calabuig JA, Negri A, Gago F, Guillen-Navarro MJ, Aviles P, Cuevas C, Garcia-Fernandez LF, Galmarini CM (2009) Molecular pharmacology and antitumor activity of Zalypsis in several human cancer cell lines. Biochem Pharmacol 78(2):162–170. doi:10.1016/j.bcp.2009.04.003
Massard C, Margetts J, Amellal N, Drew Y, Bahleda R, Stevens P, Armand JP, Calvert H, Soria JC, Coronado C, Kahatt C, Alfaro V, Siguero M, Fernandez-Teruel C, Plummer R (2013) Phase I study of PM00104 (Zalypsis(R)) administered as a 1-hour weekly infusion resting every fourth week in patients with advanced solid tumors. Invest New Drugs 31(3):623–630. doi:10.1007/s10637-012-9843-5
Griffin LB, Grohar P, Pommier Y, Khanna C, Khan J, Helman LJ (2009) ET-743 modulates EWS-FLi1 activity in Ewing’s sarcoma cells. In: Proceedings of the 100th Annual Meeting of the American Association for Cancer Research, Denver, CO, USA, 2009
Herrero A, Marco E, Garcia-Hernandez V, Martin-Castellanos C, Domingo A, Alvarez E et al (2007) Resilience of the cytotoxic effects of Zalypsis (PM00104) to the lack of functional nucleotide excision repair system. . In: Annual Meeting of the American Association for Cancer Research, 2007
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Perez-Ruixo C, Valenzuela B, Fernandez Teruel C, Gonzalez-Sales M, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2012) Population pharmacokinetics of PM00104 (Zalypsis((R))) in cancer patients. Cancer Chemother Pharmacol 69(1):15–24. doi:10.1007/s00280-011-1644-6
Olmos D, Tan DS, Jones RL, Judson IR (2010) Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: twenty years from the bench to the bedside. Cancer J 16(3):183–194. doi:10.1097/PPO.0b013e3181dbebf9
Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, Liu Q, Iorio F, Surdez D, Chen L, Milano RJ, Bignell GR, Tam AT, Davies H, Stevenson JA, Barthorpe S, Lutz SR, Kogera F, Lawrence K, McLaren-Douglas A, Mitropoulos X, Mironenko T, Thi H, Richardson L, Zhou W, Jewitt F, Zhang T, O’Brien P, Boisvert JL, Price S, Hur W, Yang W, Deng X, Butler A, Choi HG, Chang JW, Baselga J, Stamenkovic I, Engelman JA, Sharma SV, Delattre O, Saez-Rodriguez J, Gray NS, Settleman J, Futreal PA, Haber DA, Stratton MR, Ramaswamy S, McDermott U, Benes CH (2012) Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483(7391):570–575. doi:10.1038/nature11005
Conflict of interest
Pilar Lardelli, Vicente Alfaro and Mariano Siguero are employed by Pharmamar. All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, R.L., Ferrari, S., Blay, J.Y. et al. A Phase II multicenter, open-label, clinical and pharmokinetic trial of PM00104 in patients with advanced Ewing Family of Tumors. Invest New Drugs 32, 171–177 (2014). https://doi.org/10.1007/s10637-013-0037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0037-6