Summary
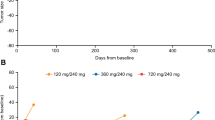
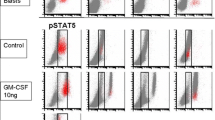
Background The kinesin spindle protein Eg5 is involved in mitosis, and its inhibition promotes mitotic arrest. EMD 534085, a potent, reversible Eg5 inhibitor, demonstrated significant preclinical antitumor activity. Methods This first-in-man, single-center, open-label, phase I dose-escalation study (3 + 3 design) investigated EMD 534085 safety, pharmacokinetics and antitumor activity in refractory solid tumors, Hodgkin’s lymphoma, or non-Hodgkin’s lymphoma. EMD 534085 (starting dose 2 mg/m2/day) was administered intravenously every 3 weeks. Doses were escalated in 100 % steps in successive cohorts of 3 patients until grade 2 toxicity occurred, followed by 50 % until the first dose-limiting toxicity (DLT) arose. If <2 of 6 patients experienced a DLT, doses were further increased by 25 %. Dose-escalation was stopped if a DLT occurred in ≥2 of 6 patients. Results Forty-four patients received EMD 534085. Median treatment duration was 43 days (range, 21–337). Thirty-eight patients (86 %) received ≥2 cycles. DLTs were grade 4 neutropenia (1 patient each at 108 and 135 mg/m2/day), and grade 3 acute coronary syndrome with troponin I elevation (1 patient at 135 mg/m2/day). The maximum tolerated dose (MTD) was 108 mg/m2/day. The most common treatment-related adverse events were asthenia (50 %) and neutropenia (32 %). EMD 534085 appeared to have linear pharmacokinetics. Increase in phospho-histone H3 positive cells in paired pre- and on-treatment biopsies showed evidence of target modulation. No complete or partial responses were observed. Best response was stable disease in 23 patients (52 %). Conclusions EMD 534085 appeared to be well tolerated; MTD was 108 mg/m2/day. Preliminary antitumor results suggested limited activity in monotherapy.




Similar content being viewed by others
References
Risinger AL, Giles FJ, Mooberry SL (2009) Microtubule dynamics as a target in oncology. Cancer Treat Rev 35:255–261
Bhat KM, Setaluri V (2007) Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res 13:2849–2854
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Sawin KE, LeGuellec K, Philippe M, Mitchison TJ (1992) Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359:540–543
Cochran JC, Sontag CA, Maliga Z, Kapoor TM, Correia JJ, Gilbert SP (2004) Mechanistic analysis of the mitotic kinesin Eg5. J Biol Chem 279:38861–38870
Schiemann K, Finsinger D, Zenke F et al (2010) The discovery and optimization of hexahydro-2H-pyrano[3,2-c]quinolines (HHPQs) as potent and selective inhibitors of the mitotic kinesin-5. Bioorg Med Chem Lett 20:1491–1495
Mardin BR, Schiebel E (2012) Breaking the ties that bind: new advances in centrosome biology. J Cell Biol 197:11–18
Tang Y, Orth JD, Xie T, Mitchison TJ (2011) Rapid induction of apoptosis during Kinesin-5 inhibitor-induced mitotic arrest in HL60 cells. Cancer Lett 310:15–24
Khoury HJ, Garcia-Manero G et al (2012) A phase 1 dose-escalation study of ARRY-520, a kinesin spindle protein inhibitor, in patients with advanced myeloid leukemias. Cancer 118:3556–3564
Gerecitano JF, Stephenson JJ, Lewis NL et al (2012) A Phase I trial of the kinesin spindle protein (Eg5) inhibitor AZD4877 in patients with solid and lymphoid malignancies. Investig New Drugs 31:355–362
Infante JR, Kurzrock R, Spratlin J et al (2012) A Phase I study to assess the safety, tolerability, and pharmacokinetics of AZD4877, an intravenous Eg5 inhibitor in patients with advanced solid tumors. Cancer Chemother Pharmacol 69:165–172
Kantarjian HM, Padmanabhan S, Stock W et al (2012) Phase I/II multicenter study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD4877 in patients with refractory acute myeloid leukemia. Investig New Drugs 30:1107–1115
Beer TM, Goldman B, Synold TW et al (2008) Southwest Oncology Group phase II study of ispinesib in androgen-independent prostate cancer previously treated with taxanes. Clin Genitourin Cancer 6:103–109
Burris HA 3rd, Jones SF, Williams DD et al (2011) A phase I study of ispinesib, a kinesin spindle protein inhibitor, administered weekly for three consecutive weeks of a 28-day cycle in patients with solid tumors. Investing New Drugs 29:467–472
Knox JJ, Gill S, Synold TW et al (2008) A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168). Investig New Drugs 26:265–272
Gomez HL, Philco M, Pimentel P et al (2012) Phase I dose-escalation and pharmacokinetic study of ispinesib, a kinesin spindle protein inhibitor, administered on days 1 and 15 of a 28-day schedule in patients with no prior treatment for advanced breast cancer. Anticancer Drugs 23:335–341
Tang PA, Siu LL, Chen EX et al (2008) Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Investig New Drugs 26:257–264
Holen K, DiPaola R, Liu G et al (2012) A phase I trial of MK-0731, a kinesin spindle protein (KSP) inhibitor, in patients with solid tumors. Investig New Drugs 30:1088–1095
Lee CW, Bélanger K, Rao SC et al (2008) A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Investig New Drugs 26:249–255
Orth JD, Tang Y, Shi J et al (2008) Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate. Mol Cancer Ther 7:3480–3489
Holen KD, Belani CP, Wilding G et al (2011) A first in human study of SB-743921, a kinesin spindle protein inhibitor, to determine pharmacokinetics, biologic effects and establish a recommended phase II dose. Cancer Chemother Pharmacol 67:447–454
Acknowledgments
Editorial and medical writing support in the preparation of this manuscript was provided by Marianne Jenal-Eyholzer, PhD, TRM Oncology, The Hague, The Netherlands, funded by Merck KGaA, Darmstadt, Germany. The clinical trial was sponsored by Merck KGaA, Darmstadt, Germany.
Ethical standards
The study was approved by the relevant ethics committee/institutional review board and was conducted in compliance with the Declaration of Helsinki as well as good clinical practice guidelines. Written informed consent was obtained from all patients before trial initiation.
Conflict of interest
ED: advisory board membership for AMGEN, Roche and IPSEN and research funding from Roche, AstraZeneca, GSK, Sanofi, Servier, IPSEN and Nanobiotics. JCS: consultancy fees from Merck Serono. FB, SK, KT, FTZ and MK: employees of Merck KGaA. AH, CM, CGR, RB, VR, CB, VL, LL, AG, AV and TdB: no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hollebecque, A., Deutsch, E., Massard, C. et al. A phase I, dose-escalation study of the Eg5-inhibitor EMD 534085 in patients with advanced solid tumors or lymphoma. Invest New Drugs 31, 1530–1538 (2013). https://doi.org/10.1007/s10637-013-0026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0026-9