Summary
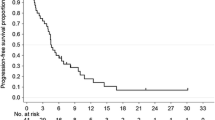
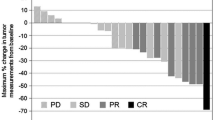
Background The aim of this study was to assess the efficacy and safety of combination regimen of capecitabine plus everolimus in patients with refractory gastric cancer who have failed to at least two cytotoxic regimens. Methods Patients received capecitabine 650 mg/m2 twice daily (D1-14) and everolimus 5 mg twice daily (D1-21) every 3 weeks until disease progression or unacceptable toxicity. The primary endpoint of the study was overall response (partial or complete response) and the secondary endpoints were progression-free survival (time between registration and disease progression or death) and overall survival. Pharmacokinetic analysis was also performed. Patients who have failed to at least two cytotoxic regimens were enrolled. Results Between March 2010 and June 2012, 47 patients were enrolled. 33 patients (70.2 %) had received more than three previous regimens prior to enrolment. Among 43 evaluable patients for treatment response, 5 patients achieved confirmed partial response and 18 patients showed stable disease, resulting in an overall response rate (ORR) of 10.6 % (95 % C.I.: 1.8–19.4 %) and disease control rate of 48.9 % (95 % C.I.:34.6–63.2 %). At a median follow-up of 106 weeks (range, 21–141 weeks), the median progression-free survival and overall survival were 11.0 weeks (95 % C.I.: 5.7–16.3 weeks) and 21.0 weeks (95 % C.I.: 14.3–27.7 weeks), respectively. Grade 3 nausea, diarrhea and stomatitis occurred in two, three and three patients, respectively. Elevated liver enzyme was observed in 21 patients and no patient had pulmonary fibrosis. Conclusions The combination of capecitabine 650 mg/m2 twice daily and everolimus 5 mg twice daily was found to be effective in a small subset of GC patients who were heavily pre-treated.

Similar content being viewed by others
References
Levi F, Lucchini F, Gonzalez JR, Fernandez E, Negri E, La Vecchia C (2004) Monitoring falls in gastric cancer mortality in Europe. Ann Oncol 15:338–345
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Tanaka M, Ma E, Tanaka H, Ioka A, Nakahara T, Takahashi H (2012) Trends of stomach cancer mortality in Eastern Asia in 1950–2004: comparative study of Japan, Hong Kong and Singapore using age, period and cohort analysis. Int J Cancer 130:930–936
Jung KW, Park S, Kong HJ et al (2012) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat 44:11–24
Van Cutsem E, Haller D, Ohtsu A (2002) The role of chemotherapy in the current treatment of gastric cancer. Gastric Cancer 5(Suppl 1):17–22
Park SJ, Hong YS, Lee JL et al (2012) Genetic polymorphisms of FcgammaRIIa and FcgammaRIIIa are not predictive of clinical outcomes after cetuximab plus irinotecan chemotherapy in patients with metastatic colorectal cancer. Oncology 82:83–89
Ji SH, Lim do H, Yi SY et al (2009) A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer 9:110
Jiang BH, Liu LZ (2008) Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Update 11:63–76
Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN (2009) The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev 35:148–159
Bjornsti MA, Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4:335–348
Cejka D, Preusser M, Woehrer A et al (2008) Everolimus (RAD001) and anti-angiogenic cyclophosphamide show long-term control of gastric cancer growth in vivo. Cancer Biol Ther 7:1377–1385
Doi T, Muro K, Boku N et al (2010) Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 28:1904–1910
Bu X, Le C, Jia F et al (2008) Synergistic effect of mTOR inhibitor rapamycin and fluorouracil in inducing apoptosis and cell senescence in hepatocarcinoma cells. Cancer Biol Ther 7:392–396
Lee KH, Hur HS, Im SA et al (2010) RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase. Cancer Lett 299:22–28
Lim T, Lee J, Lee DJ et al (2011) Phase I trial of capecitabine plus everolimus (RAD001) in patients with previously treated metastatic gastric cancer. Cancer Chemother Pharmacol 68:255–262
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Jung SH, Kim KM (2004) On the estimation of the binomial probability in multistage clinical trials. Stat Med 23:881–896
CaBT J (1983) Confidence intervals for a binomial parameter following a multistage test with application to MIL-STD 105D and medical trials. Technometrics 25:49–58
Jung SH, Owzar K, George SL, Lee T (2006) P-value calculation for multistage phase II cancer clinical trials. J Biopharm Stat 16:765–775, discussion 777–783
Atkins MB, Hidalgo M, Stadler WM et al (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909–918
Raymond E, Alexandre J, Faivre S et al (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22:2336–2347
Tabernero J, Rojo F, Calvo E et al (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610
Lang SA, Gaumann A, Koehl GE et al (2007) Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer 120:1803–1810
Fuereder T, Jaeger-Lansky A, Hoeflmayer D et al (2010) mTOR inhibition by everolimus counteracts VEGF induction by sunitinib and improves anti-tumor activity against gastric cancer in vivo. Cancer Lett 296:249–256
Yoon DH, Ryu MH, Park YS et al (2012) Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer 106:1039–1044
Van Cutsem Eea (2012) Phase III trial of everolimus (EVE) in previously treated patients with advanced gastric cancer (AGC): GRANITE-1. J Clin Oncol 30, 2012 (suppl 4; abstr LBA3)
O’Donnell A, Faivre S, Burris HA 3rd et al (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588–1595
Okamoto I, Doi T, Ohtsu A et al (2010) Phase I clinical and pharmacokinetic study of RAD001 (everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol 40:17–23
Deenen MJ, Klumpen HJ, Richel DJ et al (2012) Phase I and pharmacokinetic study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced solid malignancies. Investig New Drugs 30:1557–1565
Acknowledgments
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A102166).
Conflict of interest statement
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Su Jin Lee and Jongtae Lee contributed equally.
Rights and permissions
About this article
Cite this article
Lee, S.J., Lee, J., Lee, J. et al. Phase II trial of capecitabine and everolimus (RAD001) combination in refractory gastric cancer patients. Invest New Drugs 31, 1580–1586 (2013). https://doi.org/10.1007/s10637-013-0022-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0022-0