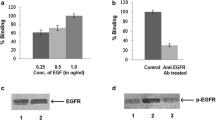
Summary
The epidermal growth factor receptor (EGFR) is a member of the HER family receptors and its activation induced by its natural ligand EGF results in colon cancer growth and progression. Panitumumab (pmAb) is a fully human IgG2 anti-EGFR antibody that blocks the EGFR actions. In the present study, we evaluated the effects of pmAb on the EGF-mediated cellular responses in a panel of colon cancer cells (HCT-8, HT-29, DLD-1 and HCT-116). HCT-1116 and DLD-1 cells showed no significant EGF-dependent cell proliferation; HT-29 and HCT-8 exhibited an EGF-dependent proliferation, with HCT-8 cells to be the most responsive with significant EGFR phosphorylation upon treatment with EGF. The effects of pmAb were then evaluated in the most EGF-responsive cells, HCT-8. In that respect, pmAb impedes the signaling cascade mediated by EGFR intracellular phosphorylation and activity of focal adhesion kinase (FAK) as well as the EGF-induced invasive and migratory potential of colon cancer cells. At the level of matrix effectors implicated in colon cancer progression we report that pmAb is a potent inhibitor of constitute and EGF-mediated gene expression of certain matrix effectors, such as membrane-type 1 metalloproteinase (MT1-MMP), extracellular metalloproteinases inducer (EMMPRIN), urokinase plasminogen activator (uPA) and syndecan-4. The obtained data demonstrated that pmAb is a specific blocker of EGF-mediated EGFR activation, resulting in a significant inhibition of colon cancer cell proliferation in early stages of growth, migration and invasiveness as well as of matrix effector implicated in cancer progression.





Similar content being viewed by others
Abbreviations
- pmAb:
-
Panitumumab
- EGF:
-
Epidermal growth factor
- EGFR:
-
EGF receptor
- ECM:
-
Extracellular matrix
- uPA:
-
Urokinase plasminogen activator
- EMPRIN:
-
Extracellular matrix metalloproteinase inducer
- FAK:
-
Focal adhesion kinase
- MMPs:
-
Matrix metalloproteinases
- MT-MMP:
-
Membrane type metalloproteinase
- PFS:
-
Progression free survival
- FBS:
-
Fetal bovine serum
References
Leserer M, Gschwind A, Ullrich A (2000) Epidermal growth factor receptor signal transactivation. IUBMB Life 49(5):405–409. doi:10.1080/152165400410254
Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G (2005) Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol 16(2):189–194. doi:10.1093/annonc/mdi057
Yarden Y (2001) The EGFR family and its ligands in human cancer: signaling mechanisms and opportunities. Eur J Cancer 37(4):S3–S8. doi:10.1016/S0959-8049(01)00230-1
Iozzo RV, Karamanos N (2010) Proteoglycans in health and disease: emerging concepts and future directions. FEBS J 277(19):3863. doi:10.1111/j.1742-4658.2010.07796.x
Nagase H, Karamanos N (2011) Metalloproteinases in health and disease: challenges and the future prospects. FEBS J 278(1):1. doi:10.1111/j.1742-4658.2010.07917.x
Hascall V, Karamanos N (2011) Regulatory roles of hyaluronan in health and disease. FEBS J 278(9):1411. doi:10.1111/j.1742-4658.2011.08068.x
Woods A, Couchman JR (2001) Syndecan 4 and focal adhesion function. Curr Opin Cell Biol 13:578–583. doi:10.1016/S0955-0674(00)00254-4
Gialeli C, Theocharis AD, Karamanos NK (2011) Matrix metalloproteinases in health and disease: roles in cancer progression and their pharmacological targeting. FEBS J 278:16–27. doi:10.1111/j.1742-4658.2010.07919.x
Stahtea NX, Roussidis AE, Kanakis I, Tzanakakis GN, Chalkiadakis G, Mavroudis D, Kletsas D, Karamanos NK (2007) Imatinib inhibits colorectal cancer cell growth and suppresses stromal-induced growth stimulation, MT1-MMP expression and pro-MMP-2 activation. Int J Cancer 121(12):2808–2814. doi:10.1002/ijc.23029
Jin JS, Wu CY, Lin YF, Wang JY, Yu CP, Sheu LF, Chiang H, Tsai WC, Lee WH (2006) Higher expression of epidermal growth factor receptor is associated with extracellular matrix metalloprotease inducer in colorectal adenocarcinoma: tissue microarray analysis of immunostaining score with clinicopathological parameters. Dis Markers 22(5–6):309–316
Menashi S, Serova M, Ma L, Vignot S, Mourah S, Calvo F (2003) Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res 63:7575–7580
Morgan H, Hill PA (2005) Human breast cancer cell-mediated bone collagen degradation requires plasminogen activation and matrix metalloproteinase activity. Canc Cell Int 5:1. doi:10.1186/1475-2867-5-1
Wu M, Rivkin A, Pham T (2008) Panitumumab: human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Therap 30(1):14–30. doi:10.1016/j.clinthera.2008.01.014
Van Custem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664. doi:10.1200/JCO.2006.08.1620
Gialeli C, Kletsas D, Mavroudis D, Kalofonos HP, Tzanakakis GN, Karamanos NK (2009) Targeting epidermal growth factor receptor in solid tumors: critical evaluation of the biological importance of therapeutic monoclonal antibodies. Curr Med Chem 16:3797–3804. doi:10.2174/092986709789177984
Nikitovic D, Pratsinis H, Berdiaki A, Gialeli C, Kletsas D, Tzanakakis GN (2012) Growth factor signaling and extracellular matrix. In: Karamanos NK (ed) Extracellular matrix: pathobiology and signaling. De Gruyter, Berlin, pp 741–753
Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P, Allal C (2006) In vivo and in vitro antitumor activity of Oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of EGFR. Canc Chemother Pharmacol 57(6):709–718. doi:10.1007/s00280-005-0123-3
Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, Augenlicht LH, Perez-Soler R, Mariadason JM (2008) PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res 68(6):1953–1961. doi:10.1158/0008-5472.CAN-07-5659
Siddiqui AD, Piperdi B (2010) KRAS mutation in colon cancer: a marker of resistance to EGFR-I therapy. Ann Surg Oncol 17(4):1168–1176. doi:10.1245/s10434-009-0811-z
Fears CY, Woods A (2006) The role of syndecans in disease and wound healing. Matrix Biol 25:443–456. doi:10.1016/j.matbio.2006.07.003
You B, Chen EX (2012) Anti-EGFR monoclonal antibodies for treatment of colorectal cancers: development of cetuximab and panitumumab. J Clin Pharmacol 52:128–155
Peeters M, Price TJ, Cervantes A et al (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28(31):4706–4713. doi:10.1177/0091270010395940
Douillard JY, Siena S, Cassidy J et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31):4697–4705. doi:10.1200/JCO.2009.27.4860
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10):1626–1634. doi:10.1200/JCO.2007.14.7116
Schwock J, Dhani N, Hedley DW (2010) Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets 14(1):77–94. doi:10.1517/14728220903460340
Hauck C, Sieg D, Hsia D, Loftus J, Gaarde W, Monia BP, Schlaepfer DD (2001) Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res 61:7079. doi:10.1242/jcs.00373
Lu Z, Jiang G, Blume-Jensen P, Hunter T (2001) Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol 21(12):4016. doi:10.1128/MCB.21.12.4016-4031.2001
Sounni NE, Noel A (2005) Membrane type-matrix metalloproteinases and tumor progression. Biochimie 87:329–342. doi:10.1016/j.biochi.2004.07.012
Weaver MA (2006) Invadopodia: specialized cell structures of cancer invasion. Clin Exp Metast 23:97–105. doi:10.1007/s10585-006-9014-1
Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S, Cao J (2011) Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biol Chem 286(38):33167–33177. doi:10.1074/jbc.M111.256644
Jo M, Thomas KS, O’Donnell DM, Gonias SL (2003) Epidermal growth factor receptor-dependent and –independent cell-signaling pathways originating from the urokinase receptor. J Biol Chem 278:1642–1646. doi:10.1074/jbc.M210877200
Liu D, Ghiso JA, Estrada Y, Ossowski L (2002) EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Canc Cell 1:445–457. doi:10.1016/S1535-6108(02)00072-7
Acknowledgements
Authors are indebted to Amgen, CA, USA for providing the active compound Panitumumab.
The support of Amgen Hellas on this research project is also acknowledged.
Conflict of interest
This research project coordinated by Dr. Nikos Karamanos has received partial funding from Amgen Hellas for the conduct of the experimental research. Dr. Karamanos is also a consultant for basic research for Amgen Hellas. These funds were paid to the Research Committee of the University of Patras.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gialeli, C., Theocharis, A.D., Kletsas, D. et al. Expression of matrix macromolecules and functional properties of EGF-responsive colon cancer cells are inhibited by panitumumab. Invest New Drugs 31, 516–524 (2013). https://doi.org/10.1007/s10637-012-9875-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9875-x