Summary
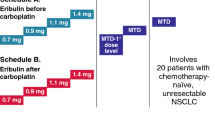
Matuzumab is a humanized IgG1 EGFR monoclonal antibody. This phase I study investigated the tolerability, safety and pharmacokinetics (PK) of matuzumab in combination with paclitaxel in patients with EGFR-expressing advanced non-small cell lung cancer (NSCLC). Six dose levels/schedules of matuzumab were explored in combination with paclitaxel. Dose was escalated from 100 mg to 1,600 mg on a modified Fibonacci scheme according to the incidence of dose-limiting toxicity (DLT) over the first two cycles. DLT was assessed in patients who completed the first two treatment cycles or who stopped treatment because of a DLT during those cycles. Patients with non-progressive disease could then continue to receive study treatment for up to 6 months. The safety population comprised 44 patients, with DLT evaluable in 33. The maximum tolerated dose was not reached, with only one DLT reported at the 1,600 mg 3-weekly dose level. The most frequent grade 3/4 adverse events across all cycles were dyspnea (23 %) and neutropenia (11 %). Matuzumab exhibited non-linear PK, with accumulation after escalation and repeated dosing. Tumor growth control was seen in 15/44 (34 %) patients, including 5/9 (56 %) at the 800 mg weekly dose level. Matuzumab combined with paclitaxel was generally well tolerated in patients with advanced NSCLC. There was some evidence of anticancer activity in relation to the matuzumab 800 mg weekly dose.
Similar content being viewed by others
References
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, Roh JK, Bajetta E, O'Byrne K, de Marinis F, Eberhardt W, Goddemeier T, Emig M, Gatzemeier U (2009) Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373:1525–1531
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Pirker R, Pereira J, von Pawel J, Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt W, Paz-Ares L, Störkel S, Schumacher K-M, von Heydebreck A, Celik I, O’Byrne K (2011) EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 13:33–42
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, Esteban E, Molinier O, Brugger W, Melezinek I, Klingelschmitt G, Klughammer B, Giaccone G (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11:521–529
Coudert B, Ciuleanu T, Park K, Wu YL, Giaccone G, Brugger W, Gopalakrishna P, Cappuzzo F (2012) Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol 23:388–394
Bria E, Milella M, Cuppone F, Novello S, Ceribelli A, Vaccaro V, Sperduti I, Gelibter A, Scagliotti GV, Cognetti F, Giannarelli D (2011) Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol 22:2277–2285
Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, Ahn MJ, Ahn JS, Suh C, Kim SW (2010) Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 16:1307–1314
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Silvestri GA, Rivera MP (2005) Targeted therapy for the treatment of advanced non-small cell lung cancer: a review of the epidermal growth factor receptor antagonists. Chest 128:3975–3984
O'Byrne KJ, Gatzemeier U, Bondarenko I, Barrios C, Eschbach C, Martens UM, Hotko Y, Kortsik C, Paz-Ares L, Pereira JR, von Pawel J, Ramlau R, Roh JK, Yu CT, Stroh C, Celik I, Schueler A, Pirker R (2011) Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol 12:795–805
Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K (2007) Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci 98:1275–1280
Pao W, Chmielecki J (2010) Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 10:760–774
Steiner P, Joynes C, Bassi R, Wang S, Tonra JR, Hadari YR, Hicklin DJ (2007) Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res 13:1540–1551
Schittenhelm MM, Kollmannsberger C, Oechsle K, Harlow A, Morich J, Honecker F, Kurek R, Storkel S, Kanz L, Corless CL, Wong KK, Bokemeyer C, Heinrich MC (2009) Molecular determinants of response to matuzumab in combination with paclitaxel for patients with advanced non-small cell lung cancer. Mol Cancer Ther 8:481–489
Schmiedel J, Blaukat A, Li S, Knochel T, Ferguson KM (2008) Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell 13:365–373
Meira DD, Nobrega I, de Almeida VH, Mororo JS, Cardoso AM, Silva RL, Albano RM, Ferreira CG (2009) Different antiproliferative effects of matuzumab and cetuximab in A431 cells are associated with persistent activity of the MAPK pathway. Eur J Cancer 45:1265–1273
Bier H, Hoffmann T, Haas I, van Lierop A (1998) Anti-(epidermal growth factor) receptor monoclonal antibodies for the induction of antibody-dependent cell-mediated cytotoxicity against squamous cell carcinoma lines of the head and neck. Cancer Immunol Immunother 46:167–173
Vanhoefer U, Tewes M, Rojo F, Dirsch O, Schleucher N, Rosen O, Tillner J, Kovar A, Braun AH, Trarbach T, Seeber S, Harstrick A, Baselga J (2004) Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol 22:175–184
Graeven U, Kremer B, Sudhoff T, Killing B, Rojo F, Weber D, Tillner J, Unal C, Schmiegel W (2006) Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. Br J Cancer 94:1293–1299
Rao S, Starling N, Cunningham D, Benson M, Wotherspoon A, Lupfert C, Kurek R, Oates J, Baselga J, Hill A (2008) Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br J Cancer 99:868–874
Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, Valladares-Ayerbes M, Wilke H, Archer C, Kurek R, Beadman C, Oates J (2010) Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol 21:2213–2219
Schiller JH, von Pawel J, Schutt P, Ansari RH, Thomas M, Saleh M, McCroskey RD, Pfeifer W, Marsland TA, Kloecker GH, Sebastian M, Pirker R, Kurek R, Beadman C, Socinski MA (2010) Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol 5:1977–1985
Kollmannsberger C, Schittenhelm M, Honecker F, Tillner J, Weber D, Oechsle K, Kanz L, Bokemeyer C (2006) A phase I study of the humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody EMD 72000 (matuzumab) in combination with paclitaxel in patients with EGFR-positive advanced non-small-cell lung cancer (NSCLC). Ann Oncol 17:1007–1013
Harandi A, Zaidi AS, Stocker AM, Laber DA (2009) Clinical Efficacy and Toxicity of Anti-EGFR Therapy in Common Cancers. J Oncol 2009:567486
Van Cutsem E, Siena S, Humblet Y, Canon JL, Maurel J, Bajetta E, Neyns B, Kotasek D, Santoro A, Scheithauer W, Spadafora S, Amado RG, Hogan N, Peeters M (2008) An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 19:92–98
Perez-Soler R (2006) Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8(Suppl 1):S7–S14
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, Ramlau R, Janaskova T, Vansteenkiste J, Strausz J, Manikhas GM, Von Pawel J (2007) Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 25:1545–1552
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892–5899
Acknowledgements
The study sponsor, Merck KGaA, was responsible for data management and statistical analysis. The clinical study was designed by Merck KGaA (Global Clinical Development Unit Oncology and Department of Biostatistics) in collaboration with CB. JTH had the final responsibility for the decision to submit the manuscript for publication. Jim Heighway of Cancer Communications and Consultancy Ltd (Knutsford, UK), provided medical writing services, which were funded by the sponsor.
Conflict of interest statement
JTH received honoraria in relation to lectures and advisory boards. SH is a salaried employee of Merck KGaA. CB has served as speaker and chairman for symposia of Merck KGaA as well as on advisory boards and his department has received research funding from Merck KGaA. CK, IC, FM and MMS declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartmann, J.T., Kollmannsberger, C., Cascorbi, I. et al. A phase I pharmacokinetic study of matuzumab in combination with paclitaxel in patients with EGFR-expressing advanced non-small cell lung cancer. Invest New Drugs 31, 661–668 (2013). https://doi.org/10.1007/s10637-012-9856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9856-0