Summary
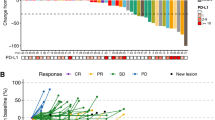
Background This study evaluated efficacy and safety of pemetrexed in patients with refractory soft tissue sarcoma. Methods Patients received pemetrexed intravenously at a dose of 500 mg/m² every 21 days until progression or unacceptable toxicity. The primary endpoint was objective tumor response. Results Fourty-eight of 53 screened patients were included and received a total of 200 cycles (median 2; range 1–30). Median age was 53 years (range, 20–81). The observed toxicity profile was favorable. NCI-CTC hematologic grade 3/4 toxicity consisted of neutropenia in 13 %, anemia in 15 %, and febrile neutropenia in 4 % of patients of patients, respectively. Non-hematologic CTC grade 3/4 toxicity consisted of elevated ASAT/ALAT in 10 %, hyperglycemia in 6 %, infection with or without neutropenia in 6 %, nausea in 2 % and stomatitis in 2 % of patients. No other grade 3 toxicities and no treatment-related toxic deaths were observed. Overall response as defined by RECIST was 5 %, 16 patients experienced stable disease (40 %). The estimated 3- and 6-months progression-free rates were 33.3 % and 14.6 %, respectively. Conclusions In patients with refractory STS, pemetrexed is well tolerated and moderately effective. The confirmed objective response rate in STS is low, however, disease stabilizations are seen in a high proportion of patients (ClinicalTrials.gov NCT00427466).



Similar content being viewed by others
References
Fletcher CD (2006) The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 48:3–12
Bauer S, Hartmann JT (2006) Locally advanced and metastatic sarcoma (adult type) including gastrointestinal stromal tumors. Crit Rev Oncol Hematol 60:112–130
Yang JC, Chang AE, Baker AR et al (1998) Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197–203
Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113:573–581
Demetri GD, Elias AD (1995) Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 9:765–785
Kopp HG, Patel S, Brucher B, Hartmann JT (2008) Potential combination chemotherapy approaches for advanced adult-type soft-tissue sarcoma. Am J Clin Dermatol 9:207–217
Grenader T, Goldberg A, Hadas-Halperin I, Gabizon A (2009) Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20:15–20
Judson I, Radford JA, Harris M et al (2001) Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 37:870–877
Hartmann JT, Patel S (2005) Recent developments in salvage chemotherapy for patients with metastatic soft tissue sarcoma. Drugs 65:167–178
Chugh R, Wathen JK, Maki RG et al (2009) Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol 27:3148–3153
Dileo P, Morgan JA, Zahrieh D et al (2007) Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 109:1863–1869
Hartmann JT, Mayer F, Schleicher J et al (2007) Bendamustine hydrochloride in patients with refractory soft tissue sarcoma: a noncomparative multicenter phase 2 study of the German sarcoma group (AIO-001). Cancer 110:861–866
Hartmann JT, Oechsle K, Huober J et al (2006) An open label, non-comparative phase II study of gemcitabine as salvage treatment for patients with pretreated adult type soft tissue sarcoma. Invest New Drugs 24:249–253
Kopp HG, Kanz L, Hartmann JT (2006) Complete remission of relapsing high-grade angiosarcoma with single-agent metronomic trofosfamide. Anticancer Drugs 17:997–998
Maki RG, Wathen JK, Patel SR et al (2007) Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol 25:2755–2763
Reichardt P, Oechsle K, Pink D et al (2003) An open label, non-comparative phase II study of topotecan as salvage treatment for patients with soft tissue sarcoma. Invest New Drugs 21:481–486
Demetri GD, Chawla SP, von Mehren M et al (2009) Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 27:4188–4196
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Simon R (1989) Optimal two-stage designs for Phase II clinical trials. Control Clin Trials 10:1–10
García-Del-Muro X, López-Pousa A, Maurel J et al (2011) Spanish Group for Research on Sarcomas. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 29:2528–2533
Chawla SP, Staddon AP, Baker LH, Schuetze SM et al (2012) Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol 30:78–84
Taylor BS, Barretina J, Maki RG et al (2011) Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer 11:541–557
Acknowledgements
This study received funding from Lilly Deutschland GmbH, Germany.
Ethical standards
This research complies with the current laws of Germany and all international regulatory guidelines for clinical trial research have been met.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartmann, J.T., Bauer, S., Egerer, G. et al. Pemetrexed in patients with refractory soft tissue sarcoma: A non-comparative multicenter phase II study of the German Sarcoma Group AIO-STS 005. Invest New Drugs 31, 167–174 (2013). https://doi.org/10.1007/s10637-012-9840-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9840-8