Summary
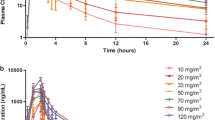
Panobinostat (LBH589) is a potent pan-histone deacetylase inhibitor. As a result of promising preclinical data, Phase I and II clinical trials of intravenous and oral panobinostat have been conducted in patients with a wide variety of hematologic and solid tumors. This is the first report of a phase I study to evaluate intravenous panobinostat given on days 1 and 8 of a 21-day cycle in patients with solid tumors. The primary objective was to characterize the safety and tolerability of panobinostat by evaluating the occurrence of dose-limiting toxicity (DLT) and determining the maximum tolerated dose (MTD) in Japanese patients with advanced solid tumors. Secondary objectives included characterizing the pharmacokinetics and assessing antitumor activity. Fourteen patients were assigned to three dose levels (Cohort 1: 10 mg/m2 [three patients], Cohort 2: 15 mg/m2 [three patients], Cohort 3: 20 mg/m2 [eight patients]), according to a standard “3 + 3” design. One patient who received 20 mg/m2 had a DLT (grade 3 elevation of γ-glutamyl transpeptidase for >7 days). Thrombocytopenia was observed in all patients (grade 3 or 4 in 8), the severity of which was dependent on the dose and platelet count at baseline. The thrombocytopenia rapidly resolved within 8 days. Plasma panobinostat levels increased dose dependently, without clinically significant drug accumulation. Stable disease for ≥4 months was observed in six patients; however, there were no complete or partial responses. It is feasible to conclude that 20 mg/m2 was the MTD and recommend as the starting dose for phase II clinical trials.


Similar content being viewed by others
References
Prince HM, Bishton MJ, Johnstone RW (2009) Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol 5:601–612
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): Successes and challenges. Cancer Lett 280:233–241
Haberland M, Montgimery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature 10:32–42
Lane AA, Chabner BA (2009) Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 27:5459–5468
Sharma S, Vogelzang NJ, Beck J et al (2007) Phase Ι pharmacokinetic (PK) and pharmacodynamic (PD) study of LBH589, a novel deacetylase (DAC) inhibitor given intravenously on a new once weekly schedule. J Clin Oncol 25:613s [abstract 14019]
Giles F, Fischer T, Cortes J et al (2006) A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 12:4628–4635
Giver CR, Jaye DL, Waller EK, Kaufman JL, Lonial S (2011) Rapid recovery from panobinostat (LBH589)-induced thrombocytopenia in mice involves a rebound effect of bone marrow megakaryocytes. Leukemia 25:362–365
Matsuoka H, Unami A, Fujimura T et al (2007) Mechanisms of HDAC inhibitor-induced thrombocytopenia. Eur J Pharmacol 571:88–96
Bishton MJ, Harrison SJ, Martin BP et al (2011) Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood 117:3658–3668
Fukutomi A, Hatake K, Matsui K et al (2011) A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs. doi:10.1007/s10637-011-9666-9
Ramalingam SS, Kummar S, Sarantopoulos J et al (2010) Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a national cancer institute organ dysfunction working group study. J Clin Oncol 28:4507–4512
Nakatani F, Tanaka K, Sakimura R et al (2003) Identification of p21WAF1/CIP1 as a direct target of EWS-Fli 1 oncogenic fusion protein. J Biol Chem 278:15105–15115
Sakimura R, Tanaka K, Nakatani F et al (2005) Antitumor effect of histone deacetylase inhibitor on Ewing’s family tumors. Int J Cancer 116:784–792
Owen LA, Kowalewski AA, Lessnick SL (2008) EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS One 3:e1965
Richter GHS, Plehm S, Fasan A et al (2009) EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci USA 106:5324–5329
Acknowledgments
This study was supported by research funding from Novartis Pharma (study No. CLBH589A1101). Hiromi Tanii and Junko Kimura are employees of Novartis Pharma. The other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial is registrated at www.clinicaltrials.gov as NCT00739414.
Rights and permissions
About this article
Cite this article
Morita, S., Oizumi, S., Minami, H. et al. Phase I dose-escalating study of panobinostat (LBH589) Administered intravenously to Japanese patients with advanced solid tumors. Invest New Drugs 30, 1950–1957 (2012). https://doi.org/10.1007/s10637-011-9751-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9751-0