Summary
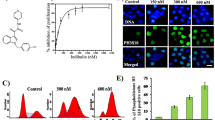
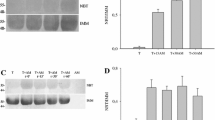
The microtubule-targeting agents derived from natural products, such as vinca-alkaloids and taxanes are an important family of efficient anti-cancer drugs with therapeutic benefits in both haematological and solid tumors. These drugs interfere with the assembly of microtubules of α/β tubulin heterodimers without altering their expression level. The aim of the present study was to investigate the effect of thymoquinone (TQ), a natural product present in black cumin seed oil known to exhibit putative anti-cancer activities, on α/β tubulin expression in human astrocytoma cells (cell line U87, solid tumor model) and in Jurkat cells (T lymphoblastic leukaemia cells). TQ induced a concentration- and time-dependent degradation of α/β tubulin in both cancer cell types. This degradation was associated with the up-regulation of the tumor suppressor p73 with subsequent induction of apoptosis. Interestingly, TQ had no effect on α/β tubulin protein expression in normal human fibroblast cells, which were used as a non-cancerous cell model. These data indicate that TQ exerts a selective effect towards α/β tubulin in cancer cells. In conclusion, the present findings indicate that TQ is a novel anti-microtubule drug which targets the level of α/β tubulin proteins in cancer cells. Furthermore, they highlight the interest of developing anti-cancer therapies that target directly tubulin rather than microtubules dynamics.



Similar content being viewed by others
References
Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agent 5:65–71. doi:10.2174/1568011053352569
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265. doi:10.1038/nrc1317
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803. doi:10.1038/nrd3253
Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH (2005) MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer 116:824–829. doi:10.1002/ijc.21013
Hopper-Borge E, Chen ZS, Shchaveleva I, Belinsky MG, Kruh GD (2004) Analysis of the drug resistance profile of multidrug resistance protein 7 (ABCC10): resistance to docetaxel. Cancer Res 64:4927–4930. doi:10.1158/0008-5472.CAN-03-3111
Canta A, Chiorazzi A, Cavaletti G (2009) Tubulin: a target for antineoplastic drugs into the cancer cells but also in the peripheral nervous system. Curr Med Chem 16:1315–1324
Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66:218–228. doi:10.1016/j.critrevonc.2008.01.008
Vats T, Buchanan G, Mehta P, Ragab A, Hvizdale E, Nitschke R, Link M, Beardsley GP, Maybee D, Krischer J (1992) A study of toxicity and comparative therapeutic efficacy of vindesine-prednisone vs. vincristine-prednisone in children with acute lymphoblastic leukemia in relapse. Invest New Drugs 10:231–234
Bellmunt J, Théodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A, Karyakin O, Delgado FM, Hurteloup P, Winquist E, Morsli N, Salhi Y, Culine S, von der Maase H (2009) Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27:4454–4461. doi:10.1200/JCO.2008.20.5534
Mielke S, Sparreboom A, Mross K (2006) Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer 42:24–30. doi:10.1016/j.ejca.2005.06.030
Galmarini CM, Kamath K, Vanier-Viornery A, Hervieu V, Peiller P, Falette N, Puisieux A, Ann Jordan M, Dumontet C (2003) Drug resistance associated with loss of p53 involves extensive alterations in microtubule composition and dynamics. Br J Cancer 88:1793–1799. doi:10.1038/sj.bjc.6600960
Alhosin M, Abusnina A, Achour M, Sharif T, Muller CM, Peluso JP, Chataigneau T, Lugnier C, Schini-Kerth VB, Bronner C, Fuhrmann G (2010) Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol 79:1251–1260. doi:10.1016/j.bcp.2009.12.015
Alhosin M, Sharif T, Mousli M, Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB, Bronner C (2011) Down-regulation of UHRF1, associated with re-expression of tumor suppressor genes, is a common feature of natural compounds exhibiting anti-cancer properties. J Exp Clin Cancer Res 30:41. doi:10.1186/1756-9966-30-41
Abusnina A, Alhosin M, Keravis T, Muller CM, Fuhrmann G, Bronner C, Lugnier C (2011) Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal 23:152–160. doi:10.1016/j.cellsig.2010.08.015
Banerjeea S, Padhyea S, Azmia A, Wanga Z, Philipa PA, Kucukb O, Sarkara FH, Mohammada RM (2010) Review on Molecular and Therapeutic Potential of Thymoquinone in Cancer. Nutrition and Cancer 62:938–946. doi:10.1080/01635581.2010.509832
Gali-Muhtasib H, Roessner A, Schneider-Stock R (2006) Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol 38:1249–1253. doi::10.1016/j.biocel.2005.10.009
Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK (2003) In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol 22:107–113
El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA (2005) Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer 117:409–417. doi:10.1002/ijc.21205
Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, Schneider-Stock R, Gali-Muhtasib H (2007) Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther 6:160–169. doi:10.4161/cbt.6.2.3575
Irwin MS, Kaelin WG (2001) Role of the newer p53 family proteins in malignancy. Apoptosis 6:17–29. doi:10.1023/A:1009663809458
Melino G, De Laurenzi V, Vousden KH (2002) p73: Friend or foe in tumorigenesis. Nat Rev Cancer 2:605–615. doi:10.1038/nrc861
Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, Frolova EI, Kovriga I, Gudkov AV, Feinstein E, Chumakov PM (2008) Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci 105:6302–6307. doi:10.1073/pnas.0802091105
Bykov VJ, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, Wiman KG (2005) PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene 24:3484–3491. doi:10.1038/sj.onc.1208419
Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK (2007) Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene 26:3473–3481. doi:10.1038/sj.onc.1210136
Peirce SK, Findley HW (2009) The MDM2 antagonist nutlin-3 sensitizes p53-null neuroblastoma cells to doxorubicin via E2F1 and TAp73. Int J Oncol 34:1395–1402. doi:10.3892/ijo_00000267
Sampath D, Calin GA, Puduvalli VK, Gopisetty G, Taccioli C, Liu CG, Ewald B, Liu C, Keating MJ, Plunkett W (2009) Specific activation of microRNA106b enables the p73 apoptotic response in chronic lymphocytic leukemia by targeting the ubiquitin ligase Itch for degradation. Blood 113:3744–3753. doi:10.1182/blood-2008-09-178707
Achour M, Jacq X, Rondé P, Alhosin M, Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A, Hughes AD, Schini-Kerth VB, Bronner C (2008) The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene 27:2187–2197. doi:10.1038/sj.onc.1210855
Schaefer KL (2008) PPARgamma Inhibitors as Novel Tubulin-Targeting Agents. PPAR Res 2008:785405. doi:10.1155/2008/785405
Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C, Schoenfeld P, Peters B, Diab-Assaf M, Pommrich U, Itani W, Lippert H, Roessner A, Schneider-Stock R (2008) Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res 68:5609–5618. doi:10.1158/0008-5472.CAN-08-0884
Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H (2010) Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res 29:87. doi:10.1186/1756-9966-29-87
Tien AL, Senbanerjee S, Kulkarni A, Mudbhary R, Goudreau B, Ganesan S, Sadler KC, Ukomadu C (2011) UHRF1 depletion causes a G2/M arrest, activation of DNA damage response and apoptosis. Biochem J 435:175–185. doi:10.1041/BJ20100840
DeLuca JG, Moree B, Hickey JM, Kilmartin JV, Salmon ED (2002) hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J Cell Biol 159:549–555. doi:10.1083/jcb.200208159
Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E (1995) Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med 1:506–526
Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K (2007) BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol 178:283–296. doi:10.1083/jcb.200702134
Kitagawa K, Niikura Y (2008) Caspase-independent mitotic death (CIMD). Cell Cycle 7:1001–1005. http://dx.doi.org/10.4161/cc.7.8.5720
Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DC, Chung FL (2009) Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem 284:17039–17051. doi:10.1074/jbc.M901789200
Harris G, Schaefer KL (2009) The microtubule-targeting agent T0070907 induces proteasomal degradation of tubulin. Biochem Biophys Res Commun 388:345–349. doi:10.1016/j.bbrc.2009.08.009
Reindl W, Yuan J, Krämer A, Strebhardt K, Berg T (2008) Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem Bio l 15:459–466. doi:10.1016/j.chembiol.2008.03.013
Effenberger-Neidnicht K, Breyer S, Mahal K, Diestel R, Sasse F, Schobert R (2011) Cellular localisation of antitumoral 6-alkyl thymoquinones revealed by an alkyne-azide click reaction and the streptavidin-biotin system. ChemBioChem 12:1237–1241. doi:10.1002/cbic.201000762
Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A (2007) Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res 21:895–897. doi:10.1002/ptr.2143
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA (2009) Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 11:373–381. doi:10.1111/j.1477-2574.2009.00059.x
Al-Majed AA, Al-Omar FA, Nagi MN (2006) Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol 543:40–47. doi:10.1016/j.ejphar.2006.05.046
Kanter M (2011) Protective effects of thymoquinone on the neuronal injury in frontal cortex after chronic toluene exposure. J Mol Histol 42:39–46. doi:10.1007/s10735-010-9305-3
Gurung RL, Lim SN, Khaw AK, Soon JF, Shenoy K, Mohamed Ali S, Jayapal M, Sethu S, Baskar R, Hande MP (2010) Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One 5:e12124. doi:10.1371/journal.pone.0012124
Acknowledgments
Abdulkhaleg Ibrahim is supported by a fellowship from the Libyan Higher Education Ministry and Tanveer Sharif from the Association pour la Recherche sur le Cancer (ARC), France. We thank Dr Dominique Wachsmann and Dr Ghada Alsaleh, University of Strasbourg, France for kindly gift for providing us normal human fibroblast cells.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
Time-course of the effects of TQ on UHRF1 expression in Jurkat cells and in normal human fibroblast cells. Jurkat cells (A) and normal human fibroblast cells (B) were exposed to 50 μM of TQ for the indicated times. Specific band of UHRF1 was detected with its expected apparent molecular weight. The data are representative of at least three independent experiments (PDF 23 kb)
Rights and permissions
About this article
Cite this article
Alhosin, M., Ibrahim, A., Boukhari, A. et al. Anti-neoplastic agent thymoquinone induces degradation of α and β tubulin proteins in human cancer cells without affecting their level in normal human fibroblasts. Invest New Drugs 30, 1813–1819 (2012). https://doi.org/10.1007/s10637-011-9734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9734-1