Summary
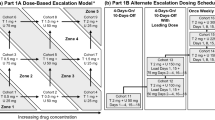
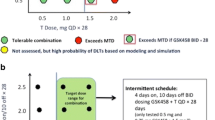
Background MKC-1 is an oral cell-cycle inhibitor with broad antitumor activity in preclinical models. Clinical studies demonstrated modest antitumor activity using intermittent dosing schedule, however additional preclinical data suggested continuous dosing could be efficacious with additional effects against the mTor/AKT pathway. The primary objectives were to determine the maximum tolerated dose (MTD) and response of continuous MKC-1. Secondary objectives included characterizing the dose limiting toxicities (DLTs) and pharmacokinetics (PK). Methods Patients with solid malignancies were eligible, if they had measurable disease, ECOG PS ≤1, and adequate organ function. Exclusions included brain metastases and inability to receive oral drug. MKC-1 was dosed twice daily, continuously in 28-day cycles. Other medications were eliminated if there were possible drug interactions. Doses were assigned using a TITE-CRM algorithm following enrollment of the first 3 pts. Disease response was assessed every 8 weeks. Results Between 5/08–9/09, 24 patients enrolled (15 M/9 F, median 58 years, range 44–77). Patients 1–3 received 120 mg/d of MKC-1; patients 4–24 were dosed per the TITE-CRM algorithm: 150 mg [n = 1], 180 [2], 200 [1], 230 [1], 260 [5], 290 [6], 320 [5]. The median time on drug was 8 weeks (range 4–28). The only DLT occurred at 320 mg (grade 3 fatigue). Stable disease occurred at 150 mg/d (28 weeks; RCC) and 320 mg/d (16 weeks; breast, parotid). Escalation halted at 320 mg/d. Day 28 pharmacokinetics indicated absorption and active metabolites. Conclusion Continuous MKC-1 was well-tolerated; there were no RECIST responses, although clinical benefit occurred in 3/24 pts. Dose escalation stopped at 320 mg/d, and this is the MTD as defined by the CRM dose escalation algorithm; this cumulative dose/cycle exceeds that determined from intermittent dosing studies. A TITE-CRM allowed for rapid dose escalation and was able to account for late toxicities with continuous dosing via a modified algorithm.
Similar content being viewed by others
References
Hanna N, Estes D, Arnott J et al (2009) Phase I/II study of MKC-1 and pemetrexed (PEM) as second-line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 27(suppl):e19005
Salazar R, Bissett D, Twelves C et al (2004) A phase I clinical and pharmacokinetic study of Ro 31-7453 given as a 7- or 14-day oral twice daily schedule every 4 weeks in patients with solid tumors. Clin Cancer Res 10:4374–82
Dupont J, Bienvenu B, Aghajanian C et al (2004) Phase I and pharmacokinetic study of the novel oral cell-cycle inhibitor Ro 31-7453 in patients with advanced solid tumors. J Clin Oncol 22:3366–74
Elser C, Hirte H, Kaizer L et al (2009) Phase II study of MKC-1 in patients with metastatic or resistant epithelial ovarian cancer or advanced endometrial cancer. J Clin Oncol 27(suppl):5577
Schneider B, Karwal M, Laufman L, et al. (2008) A phase II study of oral MKC-1 for metastatic breast cancer (MBC). J Clin Oncol 26 abstr 1046
O’Quigley J, Shen LZ (1996) Continual reassessment method: a likelihood approach. Biometrics 52:673–84
Normolle D, Lawrence T (2006) Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol 24:4426–33
Cheung YK, Chappell R (2000) Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177–82
He W, Liu J, Binkowitz B et al (2006) A model-based approach in the estimation of the maximum tolerated dose in phase I cancer clinical trials. Stat Med 25:2027–42
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–16
Team: RDC: A language and environment for statistical computing. Vienna, Austria., R Foundation for Statistical Computing, 2007
Terwogt JM, Schellens JH, Huinink WW et al (1999) Clinical pharmacology of anticancer agents in relation to formulations and administration routes. Cancer Treat Rev 25:83–101
Goodman SN, Zahurak ML, Piantadosi S (1995) Some practical improvements in the continual reassessment method for phase I studies. Stat Med 14:1149–61
Acknowledgments
The authors would like to thank the patients and families who participated, as well as the nurses and research specialists of the UWCCC Phase I Program for their efforts in conducting and managing this trial.
Funding
Supported by EntreMed, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Statement of Clinical Relevance
This manuscript describes the results of a phase I study of MKC-1 in patients with advanced solid malignancies. Preclinical and clinical studies of MKC-1 have demonstrated activity in a variety of tumors including breast and lung cancer. Here, we evaluate the safety and tolerability of continuously dosed MKC-1, which may provide additional mechanisms of antitumor activity over intermittent dosing strategies. We also describe the application of an unusual dose-escalation design and important considerations for applying reassessment models to continuous oral chemotherapy in which extended drug tolerability (in addition to toxicity) is important.
Rights and permissions
About this article
Cite this article
Tevaarwerk, A., Wilding, G., Eickhoff, J. et al. Phase I study of continuous MKC-1 in patients with advanced or metastatic solid malignancies using the modified Time-to-Event Continual Reassessment Method (TITE-CRM) dose escalation design. Invest New Drugs 30, 1039–1045 (2012). https://doi.org/10.1007/s10637-010-9629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9629-6