Summary
Myeloproliferative neoplasms (MPN) are clonal haemopoietic progenitor cell disorders characterized by the proliferation of one or more of the haemopoietic lineages (myeloid, erythroid and/or megakaryocytic). The MPNs include eight haematological disorders: chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), systemic mastocytosis (SM), chronic eosinophilic leukemia, not otherwise specified (CEL, NOS), chronic neutrophilic leukemia (CNL), and unclassifiable MPN (MPN, U). Therapeutic interventions for MPNs include the use of tyrosine kinase inhibitors (TKIs) for BCR-ABL1+ CML and JAK2 inhibitors for PV, ET and PMF. Histone deacetylase inhibitors (HDACi) are a novel class of drugs capable of altering the acetylation status of both histone and non-histone proteins, thereby affecting a repertoire of cellular functions in neoplastic cells including proliferation, differentiation, immune responses, angiogenesis and survival. Preliminary studies indicate that HDACi when used in combination with tyrosine kinase or JAK2 inhibitors may overcome resistance to the latter agents and enhance the pro-apoptotic effects on MPN cells. This review provides a review of pre-clinical and clinical studies that have explored the use of HDACi as potential therapeutics for MPNs.
Similar content being viewed by others
Introduction
Myeloproliferative neoplasms
The World Health Organization (WHO) classification of haemopoietic and lymphoid neoplasms was revised in 2008 [1]. This represented a major improvement over the 2001 WHO classification system, whereby myeloid neoplasms are now classified into five categories: acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), MDS/MPN, and myeloid and/or lymphoid malignancies associated with eosinophilia and platelet-derived growth factor receptor (PDGFR) or fibroblast growth factor receptor 1 (FGFR1) rearrangements. MPN are sub-classified into eight separate entities: chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), systemic mastocytosis (SM), chronic eosinophilic leukemia, not otherwise specified (CEL, NOS), chronic neutrophilic leukemia (CNL), and unclassifiable MPN (MPN,U).
The MPN are clonal haemopoietic stem cell disorders characterized by proliferation of one or more haemopoietic lineages. Occurrence is primarily in the 5th to 7th decade of life, however, CML and ET have been described in children. The incidence of MPN as a group is 6-10/100,000 population annually [2]. Collectively, MPN are characterised by a hypercellular bone marrow with effective haemopoiesis. Over time splenomegaly and hepatomegaly occur as a result of sequestration of blood cells and extra-medullary proliferation of haemopoietic cells. Despite an initially indolent course MPN progress over time and have the potential to transform to acute leukaemia. This manifests clinically as progressive organomegaly, worsening of peripheral cytopenias and an increasing numbers of circulating blast cells.
The diagnosis of CML requires the presence of BCR-ABL1, while its absence is required for all other MPN. CML remains the prototype for the identification and classification of myeloid neoplasms. The enhanced tyrosine kinase activity of BCR-ABL1 results in constitutive activation of a number of signal transduction pathways resulting in the leukemic phenotype observed in CML. BCR-ABL1 provided the first bona fide therapeutic target for tyrosine kinase inhibitors (TKIs). This has revolutionized the management of CML, a disorder previously fatal without allogeneic stem cell transplantation.
PV, ET, and PMF make up the non-leukemic MPN. Clonal proliferation is responsible for the overlapping expansion of erythropoietic, granulopoietic, and megakaryocytic components in the marrow and, in advanced disease, the liver and spleen. A single acquired point mutation (V617F) of the cytoplasmic Janus-associated tyrosine kinase (JAK2) occurs (heterozygous or homozygous) in the marrow and blood of almost all patients with PV and in approximately 50% of patients with ET and PMF, and is responsible for the uncontrolled myeloproliferation associated with these disorders. JAK2 is involved in transducing signals from cytokines and growth factors including erythropoietin (EPO), granulocyte-macrophage colony-stimulating factor (GM-CSF) and thrombopoietin (TPO). The mutation occurs in a highly conserved region of the pseudokinase domain that is believed to negatively regulate JAK2 signaling. In PV, the presence of JAK2 homozygosity increases with time. Additional MPN-associated molecular markers include mutations of MPL, TET2 and KIT but the diagnostic utility of MPL and TET2 mutations is limited by a low mutational frequency.
In SM, presence of the D816V KIT mutation is expected but not essential for diagnosis. CEL, NOS should be distinguished from both PDGFR-rearranged or FGFR1-rearranged neoplasms and hypereosinophilic syndrome (HES). CNL is a rare myeloproliferative disease with only 150 cases reported [1] and is characterized by sustained neutrophilia, bone marrow hypercellularity and hepatosplenomegaly. The BCR-ABL1 fusion gene is undetectable in CNL and further discrimination is required to distinguish CNL from other MPNs.
Prognosis
Prior to any effective therapy the median survival for CML was 2–3 years [3]. Subsequently, intereferon-γ (IFN-γ)-based approaches resulted in 10 year overall survival (OS) rates of approximately 25% [4]. The 10-year OS for allogeneic haemopoietic stem cell transplantation (HSCT) varied widely from 10 to 70% depending on patient age, phase of disease and donor type [5, 6]. In the current era of tyrosine kinase inhibitor (TKI) therapy 5-year OS with imatinib is 80–95% [5, 7], however, acquired resistance to imatinib in patients leads to a clinical impasse. In addition to BCR-ABL1 gene amplification resulting in overexpression of BCR-ABL1 protein, or point mutations that prevent the binding of the inhibitor to the kinase domain [8, 9], several groups have demonstrated other forms of BCR-ABL1-independent imatinib resistance [10–12]. Interestingly, BCR-ABL1-independent imatinib-resistant K652 cells display aberrant protein acetylation and increased sensitivity to histone deactylase inhibitors (HDACi) [12].
PV and ET are relatively indolent disorders that result in a modest reduction of survival, particularly evident after the first decade from diagnosis. In contrast, PMF has a more aggressive clinical course with a median survival of approximately 5 years, although younger patients with low-risk disease may experience survival in excess of 10 years [13]. With the possible exception of IFN-γ use in PV [14–16], therapy for classical MPN is aimed at ameliorating the signs and symptoms of myeloproliferation, including anaemia and/or thrombocytopenia, and to reduce the risk of thrombosis. Patients with PMF and post-ET/PV MF have progressive cytopenias, extra-medullary haemopoiesis (manifesting as splenomegaly and/or hepatomegaly), significant constitutional symptoms, the potential for blastic transformation and subsequently, premature death [17, 18]. A number of novel therapeutic strategies have been explored in these patients including farnesyl-transferase inhibition [19], proteosome inhibition [20] and immunomodulation with lenalidomide [21] but with limited efficacy. Since current therapies rarely offer more than a palliative benefit, the urgent need for improved therapeutic options for PMF and post-ET/PV MF has resulted in therapeutic targeting of the JAK2V617F mutation, a common molecular link that unifies the pathogenesis of these MPNs. A selective JAK1/2 inhibitor, INCB018424, demonstrated prolonged survival in a preclinical murine model of JAKV617F + MPN [22] and preliminary results from a phase 1/2 study resulted in objective and subjective improvements in patients with PMF and post-ET/PV MF [22]. These targeted therapeutic approaches are promising, however, other novel agents need to be explored.
Epigenetic therapy in MPN
Despite the general acceptance that genetic changes underlie the pathogenesis of cancer there is an increasing body of evidence demonstrating that in malignant cells epigenetic changes result in the abnormal transcription of structurally intact genes. In general, malignant tissue is characterized by global hypermethylation of DNA [23, 24]. In addition, malignant tissues also demonstrate increased histone deacetylation that results in chromatin condensation and transcriptional repression of tumor suppressor genes [24–26]. Collectively, these post-translational modifications of histones and DNA methylation result in transcriptional silencing of tumour suppressor and cell differentiation genes, thus promoting cell survival by blocking apoptosis and senescence. Importantly, epigenetic but not genomic changes have the potential to be modulated for therapeutic benefit. Novel therapeutic approaches are now being developed to target some of these molecular lesions, including epigenetic alterations, by making use of methyltransferase and histone deacetylase inhibitors. However, the extent and specific role of epigenetic modifications in MPN remain poorly understood. Furthermore, increased knowledge of the linkage between genetic and epigenetic changes that promote tumourogenesis and the identification of appropriate biomarkers represents a prerequisite for successful epigenetics-directed MPN therapy. The production of pro-inflammatory cytokines by stromal elements and constitutive activation of aberrant signal transduction pathways by malignant cells could result in disease progression. Indeed, the constitutive activation of the JAK/STAT pathway in JAKV617F+MPN can result in subsequent epigenetic silencing of tumour suppressor genes [27, 28], suggesting that these changes result in further evolution of the disease.
Epigenetic events in MPN are most prevalent in PMF as evidenced by genome-wide methylation studies demonstrating a high rate of methylation in samples from PMF patients [29, 30]. In addition, a decreased level of acetylation as a result of increased histone deacetylase activity has also been observed [30]. Most of the work on gene methylation in MPN has focused on a limited number of genes, in particular, the status of the suppressors of cytokine signaling family proteins (SOCS) [31–34]. The increased number of CD34-positive cells in the peripheral blood of PMF patients has been attributed to the reduced membrane expression of C-X-C chemokine receptor type 4 (CXCR4) [35, 36] which is secondary to increased methylation of the CpG islands of the CXCR4 promoter [37]. Ex vivo treatment of CD34-positive cells from PMF patients with hypomethylating agents rectified the abnormal homing and proliferation of these cells [38]. Wang et al., (2008) demonstrated increased HDAC levels in the CD34-positive cell fractions of patients with PMF [30]. Given these changes, it would seem reasonable to explore epigenetic therapy as a therapeutic option in MPN, especially PMF, where therapeutic options are currently limited. The use of DNA hypomethylation therapy has been found to be efficacious in patients with MDS leading to improvement in disease-associated cytopenias, delay in transformation to AML and improved OS [39, 40], however, the hypomethylating agent 5-azacytidine has limited efficacy in PMF [17, 41].
Histone deacetylases and inhibitors
Acetylation of proteins is modulated by the dynamic and antagonistic action of two classes of enzymes, histone deacetylases (HDACs) and histone acetyltransferases (HATs). HATs acetylate amino –terminal lysine residues whereas HDACs catalyse the removal of acetyl groups. The HDAC family comprises 18 genes, grouped into 4 classes based on their homology to yeast orthologues. Class I (HDACs 1–3 and 8), Class II A (HDAC 4,5,7 and 9), Class II B (HDAC6 and 11) and Class IV (HDAC11) require zinc for catalysing deactylase activity, while Class III (Sirutins 1–7) utilise nicotine adenine dinucleotide (NAD+) for their catalytic mechanisms [26, 42]. Moreover, there is growing evidence that the acetylation status of proteins plays a key role in the regulation of cellular signalling and disease development [27, 42–48].
HDACi represent a novel class of chemotherapeutic drugs that are able to regulate cellular functions through their ability to inhibit HDAC action and can be subdivided into six groups based on their structure: Hydroxamic acid-derived compounds, cyclic peptides, short-chain fatty acids, benzamides, electrophilic ketones and several agents that are not otherwise classifiable [49]. Their inhibitory effects on cell proliferation and survival can be mediated by both chromatin-dependent (acetylation of histone proteins) and chromatin-independent pathways (acetylation of non-histone proteins). Alterations in gene expression induced by histone acetylation results in changes in expression levels of pro- and anti-apoptotic genes and modulation of cell cycle genes (reviewed in [48, 50]). Amongst the non-histone acetylation targets that via HDACi induced acetylation could potentially impart anti-cancer effect are transcription factors (p53, c-Myc, Smad7, BCL6, GATA, STAT3, NF-κB, E2F family, MEF2, AML1, CREB), chaperone proteins (Hsp90), cytoskeletal proteins (α-tubulin), nuclear import proteins (Importin α), DNA repair enzymes (Ku70) and steroid receptors (androgen receptors, estrogen receptor) (reviewed in [46, 51, 52]). Furthermore, HDACi are able to target both proliferating and non-proliferating transformed cells to induce growth arrest and apoptosis, although the molecular mechanisms underlying the apparent specificity of HDACi to induce cytotoxicity of neoplastic cells and not normal cells remains to be elucidated [53–57].
MPN and HDAC inhibitors
BCR-ABL1+ chronic myelogenous leukemia
Chronic Myeloid Leukemia (CML) is a MPN of myeloid progenitor cells characterised by the expression of a 210-KDa fusion oncoprotein, BCR-ABL, resulting from the juxtapositioning of chromosomes 9 and 22 [the Philadelphia chromosome translocation, t(9;22)]. The BCR-ABL kinase is constitutively active and via downstream signaling regulates multiple survival pathways, including Ras/Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK), PI3/AKT, NF-κB, JAK/STAT, anti- and pro-apoptotic proteins [58]. Monotherapy with the TKI imatinib mesylate is effective in chronic-phase CML patients, however, accelerated and blast phase patients are generally resistant to imatinib [59]. Imatinib resistance is usually characterized by amplification of the BCR-ABL gene, protein overexpression, missense mutations in BCR-ABL kinase domains or through mechanisms independent of BCR-ABL signalling [10–12, 60, 61]. Dasatinib and nilotinib are two novel second generation kinase inhibitors that are effective in imatinib-resistance patients, although patients with the T315I BCR-ABL mutation do not respond to these drugs [62, 63]. Therefore, additional therapies to combat emergent TKI resistance are required and are currently being explored.
Recent studies indicate that HDACi are capable of attenuating the expression of BCR-ABL and of inducing apoptosis in both imatinib sensitive and resistant cell lines [64–69]. Furthermore, the tumour-specificity of HDACi was seen with HDACi preferentially affecting the BCR-ABL positive clonal cells and not normal cells [70–73]. In addition, analyses of the expression and functional activity of HDACs and HATs in the imatinib resistant CML cell line K562 have indicated that Class I (HDAC1, 2 and 3), Class IIA (HDAC4) and Class III (SIRT1) HDACs are all elevated and increased compared to imatinib sensitive cells, whereas the levels of HATs are decreased [12, 74]. To date, several HDACi that have been tested against CML cells include suberoylanilide hydroxamic acid (SAHA [Vorinostat]), LBH589 (Panobinostat), LBQ824, apicidin, FK-228 (Romidepsin [Depsipeptide]), valproate, sodium butryate and AN-9 (Pivanex). In most of the studies performed the primary mechanism by which HDACi appeared to induce apoptosis in CML cells was through the reduction of BCR-ABL levels and therefore downregulation of the multiple signalling pathways controlling survival of the neoplastic cells. As such, while the expression of HDACs and HATs in imatinib-responsive and refractory patient samples has not been assessed it would appear that HDAC inhibition may represent a promising anti-leukemic approach for imatinib-refractory patients.
The pan-HDAC inhibitors SAHA, LBH589 and LAQ824 display inhibitory activity against both Class I and Class II HDACs and are therefore able to modulate a diverse range of proteins involved in growth arrest and apoptosis [26, 49, 57, 75]. SAHA, one of the most investigated compounds, has been used in preclinical studies of human imatinib-sensitive and –resistant cell lines and in CD34+ cells from imatinib-refractory patients either alone or in combination with kinase inhibitors [12, 68, 70, 76, 77], proteosome inhibitors [78] or a MEK/ERK pathway inhibitor [73]. LBH589 and LAQ824 also induce cytotoxicity in imatinib-refractory CML cell lines and/or patient samples both alone and in combination with other agents [64, 65, 67]. Apicidin overcomes resistance to TNF-related apoptosis inducing ligand (TRAIL) in K562 cells via inhibition of the PI3K/AKT pathway and nuclear translocation of NF-κB with a subsequent reduction in Bcl-xL expression [79]. Likewise, FK228 induces cell cycle arrest and apoptosis of imatinib-resistant CML cells and in primary patient samples, in contrast however, expression of Bcl-xL, Bcl-2 or Bax does not change [80, 81]. In addition, Okabe et al., demonstrated that FK228 treatment induces a diverse range of histone and non-histone protein acetylation in BCR-ABL transfected cell lines, including acetylation of p53, Hsp90 and BCR-ABL [81]. Valproate, commonly used as an anti-epileptic drug, enhances imatinib-induced growth arrest and apoptosis in CML cell lines and primary mononuclear cells with concomitant p21CIP1 upregulation, Bcl-2 downregulation and interference with NF-κB DNA binding [66, 82]. Sodium butyrate (SB) or AN-9 (Pivanex) alone or in combination with the MEK inhibitor PD184352, bortezomib or imatinib results in synergistic lethality of CML cell lines [73, 78, 83, 84]. Importantly, combination treatments of TKI-resistant neoplastic cells appear to overcome drug resistance compared to treatment with HDACi alone [64–66, 68, 70, 72, 73, 76–80, 84]. Overcoming drug resistance could be attributed to either the synergistic reduction of BCR-ABL protein levels and/or conceivably the elimination of leukemia stem cells by HDACi that are not affected by conventional TKI treatment [85].
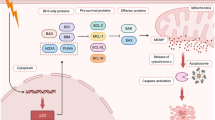
In general, HDACi induce apoptosis in neoplastic cells by modulating multiple signalling pathways that orchestrate proliferation, differentiation and survival. Their anti-proliferative effect is mediated through increased production of cell cycle inhibitor proteins p21CIP1 [64–68, 72, 73, 77, 78] and in some instances, p27KIP1 [64, 67, 68], attenuation of cyclin D1 [73, 78] and hypophosphorylation of the retinoblastoma (Rb) protein [45]. While HDACi treatment alone induces chromatin-dependent transactivation of p21CIP1 expression resulting in growth arrest, combination treatment with a MEK1/2 inhibitor or the multikinase inhibitor Sorafenib abrogates p21CIP1 induction resulting in enhanced apoptosis [73, 77]. Indeed, p21CIP1 is known to oppose apoptosis through multiple mechanisms, including inhibition of caspase-3 activation [86, 87]. Therefore, whereas HDACi mediated p21CIP1 expression induces cell cycle arrest, cell lethality may require p21CIP1 downregulation. The expression of p27KIP1 appears to be related to a non-transcriptional mechanism and possibly a consequence of BCR-ABL mediated regulation through the PI3K/AKT pathway [67, 88]. Lethality results from activation of both the extrinsic and intrinsic apoptotic pathways [37, 40, 43, 44], through production of reactive oxygen species (ROS) [71, 73, 78] and by the induction of autophagy [71]. In particular, induction of pro-apoptotic Bim and in some instances, a decrease in pro-survival Bcl-xL is critical for HDACi mediated apoptosis [64, 67, 69, 76, 79]. Finally, the proteosome inhibitor bortezomib or the MEK pathway inhibitor PD184352 when used in combination with SAHA also affects multiple signalling pathways, including induction of Jun Kinase (JNK) phosphorylation, inactivation of AKT and abrogation of NF-κB DNA binding [73, 78, 79].
Treatment with HDACi also influences gene expression through chromatin-independent mechanisms by acetylating non-histone proteins. However, studies that specifically focus on chromatin-independent targets of HDACi in CML cells are limited. Some of the non-histone HDAC substrates that are known to be involved in the cytotoxicity of CML cells post HDACi exposure are Hsp90, p53, Ku70 and α-tubulin. Importantly, imatinib-resistant K562 cells display hypoacetylation of these non-histone proteins [12, 73]. Acetylation of Hsp90, a HDAC6 substrate, results in accelerated degredation of HSP90 client proteins, including BCR-ABL, AKT and c-Raf, via the 26S proteosome [65, 76, 89–91]. Reduction of BCR-ABL levels post-HDACi treatment, observed in most cases of HDACi treated CML cells, appears to be a consequence of disrupted Hsp90 chaperone function and proteosomal degradation of BCR-ABL [12, 65, 67, 76]. Ku70, a DNA repair enzyme, is another known HDAC substrate and when deacetylated it sequesters the proapoptotic protein Bax in the cytoplasm rendering it inactive. Acetylation of Ku70 by HATs is integral to stress-induced lethality by Bax [92]. In imatinib-resistant K562 cell line, the expression of cytoplasmic Ku70 is increased, while the amount of acetylated Ku70 is markedly decreased, suggesting that Bax-mediated apopotosis is inhibited [12]. Transcriptional activation of the tumour suppressor p53 by acetylation also plays a key role in HDACi mediated cell cycle arrest and apoptosis. Deacetylation of p53 by HDAC1–3 or SIRT1 inhibits its transcriptional activity, and subsequently, the activation of its downstream molecules, Bax and p21 [93–95]. Therefore, acetylation of intracellular proteins by HDACi likely plays a critical role in orchestrating the cellular fate of leukemic cells.
‘Classic’ MPN
The safety and efficacy of HDACi in the treatment of patients with MPN has been evaluated in a small number of preclinical and phase I/II studies. The rationale for the use of HDACi in MPN stems from pre-clinical studies that have demonstrated enhanced sensitivity of JAK2V617F mutated versus non-mutated cells derived from patients with PV or PMF [96, 97]. Epigenetic therapy provides a promising platform for the treatment of PMF; evidence for this was provided by the preferential reduction in the number of JAK2V617F-positive progenitor cells when sequential administration of 5-aza-2′-deoxycytidine (a DNA methyltransferase inhibitor) and Trichostin A (a pan-HDAC inhibitor) was performed [97]. Furthermore, quantitative analysis of Class I-III HDACs mRNA expression and activity in CD34+ cells from PMF patients reveals that HDAC1, 2, 6, 8, 10 and SIRT1, 2, 3, 5 and 7 are overexpressed, while HDAC4, 5 and 11 and SIRT4 are downregulated [30]. The pattern of HDAC mRNA expression did not correlate with the JAK2V617F mutation status, furthermore, the relevance of the aberrant PMF CD34+ cell HDAC mRNA expression and activity and subsequent effects on biological function and pathogenesis of PMF are unknown.
Exposure of mutant cells to the Class I and II HDAC inhibitor, ITF2357, down-regulated JAK2V617F, p-STAT3 and p-STAT5 levels [96]. An ITF2357 Phase IIA study demonstrated efficacy in PV, ET and PMF patients with rapid improvement of constitutional symptoms, reduction in splenomegaly and improved haemopoietic function [97]. Treatment of CD34+ PMF-MPN cells with LBH589 induces apoptosis of PMF-MPN cells and inhibits the expression and activity of JAK2V617F with a subsequent reduction in p-STAT3, p-STAT5, p-AKT, p-GATA-1 and Bcl-xL [98]. In addition, the binding of HSP90 to JAK2 was partially inhibited by LBH589 treatment suggesting that acetylation of HSP90 could be playing a role in the degradation of JAK2 protein. Synergistic induction of MPN cell apoptosis was also observed when LBH589 and a JAK2 inhibitor (TG101209) were combined [98]. A Phase IA/II trial evaluating oral LBH589 in patients with PMF (n = 10) and post-PV MF (n = 3) that included 9 patients with JAK2V617F mutation exhibited clinical activity for up to 39 months including a reduction in spleen size and improvement in constitutional symptoms [99]. Similarly, a Phase I study by Mascarenhas et al. of 12 patients with PMF or post-ET/PV MF demonstrated clinical efficacy in 6 patients (regardless of JAK2V617F mutation status) with minimal toxicity [100]. In addition, a single case report has described an improvement in cytopenias in a patient with post-ET MF with the use of vorinostat [101].
Conclusion
Therapeutic interventions for MPNs include the use of highly targeted and efficacious therapy with TKIs for the abnormal BCR-ABL fusion protein found in CML and the use of less specific agents, including JAK2 inhibitors, that target ubiquitously expressed proteins in JAKV617F mutated PV, ET and PMF. Despite TKIs revolutionising the management of CML, TKI resistance can occur through BCR-ABL-independent mechanisms that may at least in part be due to abnormal acetylation of non-histone proteins. Furthermore, abnormal histone deacetylase activity has been noted in PMF and provides a rationale for the treatment of MPN with HDACi, either alone or in combination with existing therapies. Preliminary studies indicate that HDACi when used in combination with TKIs or JAK2 inhibitors may overcome drug resistance and enhance the apoptotic effects on MPN cells. HDACi likely induce lethality through a combination of both epigenetic (chromatin-dependent) and non-epigenetic (chromatin-independent) changes. Importantly, epigenetic but not genomic changes have the potential to be modulated for therapeutic benefit, however, the extent and specific role of epigenetic aberrations such as histone deacetylase activity in MPN are still poorly understood. Increased knowledge of the linkage of genetic and epigenetic changes that promote tumorogenesis represents a prerequisite for successful epigenetic-directed MPN therapy. Further pre-clinical and phase I/II studies are required to ascertain the utility of HDACi in the management of selected individuals with MPN.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) World health organisation classification of tumours of haemopoietic and lymphoid tissues. International Agency for Research on Cancer (IARC) Press, Lyon
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer Statistics, 2007. CA Cancer J Clin 57:43–66
Geary CG (2000) The story of chronic myeloid leukaemia. Br J Haematol 110:2–11
Baccarini M, Russo D, Rosti G, Martinelli G (2003) Interferon-alfa for chronic myeloid leukemia. Semin Hematol 40:22–33
Baccarini M, Saglio G, Goldman L et al (2006) Evolving concepts in the management of chronc myeloid leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood 108:1809–1820
Gratwohl A, Brand R, Apperley J et al (2006) Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 91:513–521
Druker BJ, Guilhot F, O’Brien SG et al (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355:2408–2417
Kantarjian HM, Talpaz M, Giles F, O’Brien S, Cortes J (2006) New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann Intern 145:913–923
Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD (2007) Second generation inhibitors of BCR-ABL for the treatment of imatinib resistant chronic myeloid leukaemia. Nat Rev Cancer 7:345–256
Dai Y et al (2004) A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J Biol Chem 279(33):34227–34239
Donato NJ et al (2004) Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res 64(2):672–677
Lee SM et al (2007) Bcr-Abl-independent imatinib-resistant K562 cells show aberrant protein acetylation and increased sensitivity to histone deacetylase inhibitors. J Pharmacol Exp Ther 322(3):1084–1092
Vannucchi AM, Guglielmelli P, Rambaldi A, Bogani C, Barbui T (2009) Epigenetic therapy in myeloproliferative neoplasms: evidence and perspectives. J Cell Mol Med 13:1437–1450
Jones AV, Silver RT, Waghorn et al (2006) Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood 107:3339–3341
Kiladjian JJ, Chomienne C, Fenaux P (2008) Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia 122:1990–1998
Kiladjian JJ, Cassinat B, Chevret S et al (2008) Pegylated Interferon-alfa-2a induces complete haematological and molecular responses with low toxicity in polycythemia vera. Blood 112:3065–3072
Mesa RA, Verstovek S, Rivera C et al (2009) 5-Azacitidine has limited therapeutic activity in myelofibrosis. Leukemia 23:180–182
Cervantes F, Mesa R, Barosi G (2007) New and old treatment modalities in primary myelofibrosis. Cancer J 13:377–383
Mesa RA, Camoriano JK, Geyer SM et al (2007) A phase II trial of tifarnib in myelofibrosis: primary, post-polycythemia vera and post-essential thrombocythemia. Leukemia 21:1964–1970
Mesa RA, Verstovsek S, Rivera C et al (2008) Bortezomib therapy in myelofibrosis: a phase II clinical trial. Leukemia 22:1636–1638
Tefferi A, Lasho TL, Mesa RA et al (2007) Lenalidomide therapy in del(5)(q31)-associated myelofibrosis: cytogenetic and JAK2V617F molecular remissions. Leukemia 21:1827–1828
Quintas-Cardama A, Vaddi K, Liu P et al (2010) Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115:3109–3117
Esteller M (2007) Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet 16(Spec No 1):50–59
Fraga MF et al (2005) Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37(4):391–400
Witt O et al (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277(1):8–21
Timmermann S et al (2001) Histone acetylation and disease. Cell Mol Life Sci 58(5–6):728–736
Shi S, Calhoun HC, Xia F et al (2006) JAK signalling globally counteracts heterochromatic gene silencing. Nat Genet 38:1071–1076
Zhang Q, Wang HY, Woetmannn A et al (2006) STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T cells. Blood 108:1058–1064
Tefferi A (2008) Epigenetic alterations and anti-epigenetic therapy in myelofibrosis. Leuk Lymphoma 49:2231–2232
Wang JC et al (2008) Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk Lymphoma 49(12):2321–2327
Fourcouclas N, Li J, Gilby DC et al (2008) Methylation of the suppressor of cytokine signalling 3 gene (SOCS3) in myeloproliferative disorders. Haematologica 93:1635–1644
Capello D, Deambrogi C, Rossi D et al (2008) Epigenetic inactivation of suppressors of cytokine signalling in Philadelphia-negative chronic myeloproliferative disorders. Br J Haematol 141:504–511
Fernandez-Mercado M, Cebrian V, Euba B et al (2008) Methylation status of SOCS1 and SOCS3 in BCR-ABL negative and JAK2V617F negative chronic myeloproliferative neoplasms. Leuk Res 32:1638–1640
Jost E, do ON, Dahl E (2007) Epigenetic alterations complement mutations of JAK2 tyrosine kinase in patients with BCR/ABL-negative myeloproliferative disorders. Leukemia 21:505–510
Guglielmelli P, Zini R, Bogani C et al (2007) Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms’ tumor gene 1 (WT1). Stem Cells 25:165–173
Rosti V, Massa M, Vannucchi AM et al (2007) The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis 38:280–286
Bogani C, Ponziani V, Gugliemelli P et al (2008) Hypermethylation of CXCR4 promoter in the CD34+ cells from patients with primary myelofibrosis. Stem Cells 26:1920–1930
Wang X, Zhang W, Ishii T et al (2008) Correction of the abnormal trafficking of primary myelofibrosis CD34+ cells by treatment with chromatin modifying agents. Blood 112:101A
Silverman LR, Demakos EP, Peterson BL et al (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20:2429–2440
Fenaux P, Mufti GJ, Santini V et al (2007) Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional care regimens (CCR). Blood 110:817
Quintas-Cardama A, Tong W, Kantarjian H et al (2008) A phase II study of 5-Azacitidine for patients with primary and post essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia 22:965–970
Haberland M et al (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10(1):32–42
Haberland M et al (2009) Genetic dissection of histone deacetylase requirement in tumor cells. Proc Natl Acad Sci USA 106(19):7751–7755
Lehrmann H et al (2002) Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv Cancer Res 86:41–65
Mai A et al (2005) Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med Res Rev 25(3):261–309
Spange S et al (2009) Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol 41(1):185–198
Glozak MA et al (2005) Acetylation and deacetylation of non-histone proteins. Gene 363:15–23
Glozak MA et al (2007) Histone deacetylases and cancer. Oncogene 26(37):5420–5432
Miller TA et al (2003) Histone deacetylase inhibitors. J Med Chem 46(24):5097–5116
Bolden JE et al (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5(9):769–784
Minucci S et al (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6(1):38–51
Xu WS et al (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26(37):5541–5552
Brinkmann H et al (2001) Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts. J Biol Chem 276(25):22491–22499
Burgess A et al (2004) Histone deacetylase inhibitors specifically kill nonproliferating tumour cells. Oncogene 23(40):6693–6701
Burgess AJ et al (2001) Up-regulation of p21(WAF1/CIP1) by histone deacetylase inhibitors reduces their cytotoxicity. Mol Pharmacol 60(4):828–837
Qiu L et al (2000) Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 11(6):2069–2083
Insinga A et al (2005) Mechanisms of selective anticancer action of histone deacetylase inhibitors. Cell Cycle 4(6):741–743
Deininger MW et al (2000) BCR-ABL tyrosine kinase activity regulates the expression of multiple genes implicated in the pathogenesis of chronic myeloid leukemia. Cancer Res 60(7):2049–2055
Savage DG et al (2002) Imatinib mesylate–a new oral targeted therapy. N Engl J Med 346(9):683–693
Gorre ME et al (2001) Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293(5531):876–880
Shah NP et al (2002) Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2(2):117–125
Aguilera DG et al (2009) Dasatinib in chronic myeloid leukemia: a review. Ther Clin Risk Manag 5(2):281–289
Swords R et al (2009) Nilotinib: optimal therapy for patients with chronic myeloid leukemia and resistance or intolerance to imatinib. Drug Des Devel Ther 3:89–101
Fiskus W et al (2006) Combined effects of novel tyrosine kinase inhibitor AMN107 and histone deacetylase inhibitor LBH589 against Bcr-Abl-expressing human leukemia cells. Blood 108(2):645–652
George P et al (2005) Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood 105(4):1768–1776
Morotti A et al (2006) Valproate enhances imatinib-induced growth arrest and apoptosis in chronic myeloid leukemia cells. Cancer 106(5):1188–1196
Nimmanapalli R et al (2003) Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res 63(16):5126–5135
Nimmanapalli R et al (2003) Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood 101(8):3236–3239
Xu Y et al (2005) The histone deacetylase inhibitor suberoylanilide hydroxamic acid down-regulates expression levels of Bcr-abl, c-Myc and HDAC3 in chronic myeloid leukemia cell lines. Int J Mol Med 15(1):169–172
Dai Y et al (2008) Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood 112(3):793–804
Carew JS et al (2007) Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110(1):313–322
Fiskus W et al (2006) Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin Cancer Res 12(19):5869–5878
Yu C et al (2005) Synergistic interactions between MEK1/2 and histone deacetylase inhibitors in BCR/ABL+ human leukemia cells. Leukemia 19(9):1579–1589
Kalle AM et al (2010) Bcr-Abl-independent mechanism of resistance to imatinib in K562 cells: Induction of cyclooxygenase-2 (COX-2) by histone deacetylases (HDACs). Leuk Res
Rasheed WK et al (2007) Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs 16(5):659–678
Fiskus W et al (2008) Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res 14(19):6106–6115
Dasmahapatra G et al (2007) Synergistic interactions between vorinostat and sorafenib in chronic myelogenous leukemia cells involve Mcl-1 and p21CIP1 down-regulation. Clin Cancer Res 13(14):4280–4290
Yu C et al (2003) The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood 102(10):3765–3774
Park SJ et al (2009) Cotreatment with apicidin overcomes TRAIL resistance via inhibition of Bcr-Abl signaling pathway in K562 leukemia cells. Exp Cell Res 315(11):1809–1818
Kawano T et al (2004) Depsipeptide enhances imatinib mesylate-induced apoptosis of Bcr-Abl-positive cells and ectopic expression of cyclin D1, c-Myc or active MEK abrogates this effect. Anticancer Res 24(5A):2705–2712
Okabe S et al (2007) Depsipeptide (FK228) preferentially induces apoptosis in BCR/ABL-expressing cell lines and cells from patients with chronic myelogenous leukemia in blast crisis. Stem Cells Dev 16(3):503–514
Kircher B et al (2009) Anti-leukemic activity of valproic acid and imatinib mesylate on human Ph+ALL and CML cells in vitro. Eur J Haematol 83(1):48–56
Grebenova D et al (2006) The proteomic study of sodium butyrate antiproliferative/cytodifferentiation effects on K562 cells. Blood Cells Mol Dis 37(3):210–217
Rabizadeh E et al (2007) Pivanex, a histone deacetylase inhibitor, induces changes in BCR-ABL expression and when combined with STI571, acts synergistically in a chronic myelocytic leukemia cell line. Leuk Res 31(8):1115–1123
Zhang B et al (2010) Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell 17(5):427–442
Bissonnette N et al (1998) p21-induced cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase II blockage. Oncogene 16(26):3461–3469
Suzuki A et al (1999) Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene 18(5):1239–1244
Gesbert F et al (2000) BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J Biol Chem 275(50):39223–39230
Bali P et al (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280(29):26729–26734
Rao R et al (2008) HDAC6 inhibition enhances 17-AAG–mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood 112(5):1886–1893
Cohen HY et al (2004) Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 13(5):627–638
Juan LJ et al (2000) Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem 275(27):20436–20443
Kume S et al (2006) Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med 40(12):2175–2182
Wang LG et al (2008) De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int J Oncol 33(2):375–380
Guerini V et al (2008) The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F). Leukemia 22(4):740–747
Shi J et al (2007) Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res 67(13):6417–6424
Rambaldi A, Dellacasa CM, Salmoiraghi S et al (2008) A phase 2A study of the histone-deacetlase inhibitor ITF2357 in patients with Jak2V617F positive chronic myeloproliferative neoplasms. 112:110A
Wang Y et al (2009) Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood 114(24):5024–5033
DeAngelo DJ, Spencer A, Fischer T et al (2009) Activity of oral panobinostat (LBH589) in patients with myelofibrosis. Blood 114:2898
Mascareenhas J, Wang X, Rodriguez A (2009) A phase I study of LBH589, a novel histone deacetylase inhibitor in patients with primary myelofibrosis (PMF) and post-polycythemia/essential thrombocythemia myelofibrosis (Post-PV/ET MF). Blood 114:308
Lee J (2009) Clinical efficacy of vorinostat in a patient with essential thrombocytosis and subsequent myelofibrosis. Ann Hematol 88(7):699–700
Conflicts of Interest
Sridurga Mithraprabhu, George Grigoriadis, Tiffany Khong: No conflicts to report.
Andrew Spencer: Received research funding from MSD and Novartis. Received honoraria from Novartis.
Logistical support during submission of this article was provided by Springer Healthcare LLC. This support was funded by Novartis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mithraprabhu, S., Grigoriadis, G., Khong, T. et al. Deactylase inhibition in myeloproliferative neoplasms. Invest New Drugs 28 (Suppl 1), 50–57 (2010). https://doi.org/10.1007/s10637-010-9590-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9590-4