Summary
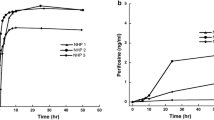
Purpose Sorafenib is a small molecule inhibitor of multiple signaling kinases thought to contribute to the pathogenesis of many tumors including brain tumors. Clinical trials with sorafenib in primary and metastatic brain tumors are ongoing. We evaluated the plasma and cerebrospinal fluid (CSF) pharmacokinetics (PK) of sorafenib after an intravenous (IV) dose in a non-human primate (NHP) model. Methods 7.3 mg/kg of sorafenib free base equivalent solubilized in 20% cyclodextrin was administered IV over 1 h to three adult rhesus monkeys. Serial paired plasma and CSF samples were collected over 24 h. Sorafenib was quantified with a validated HPLC/tandem mass spectrometry assay. PK parameters were estimated using non-compartmental methods. CSF penetration was calculated from the AUCCSF : AUCplasma. Results Peak plasma concentrations after IV dosing ranged from 3.4 to 7.6 μg/mL. The mean ± standard deviation (SD) area under the plasma concentration from 0 to 24 h was 28 ± 4.3 μg•h/mL, which is comparable to the exposure observed in humans at recommended doses. The mean ± SD clearance was 1.7 ± 0.5 mL/min/kg. The peak CSF concentrations ranged from 0.00045 to 0.00058 μg/mL. The mean ± SD area under the CSF concentration from 0 to 24h was 0.0048 ± 0.0016 μg•h/mL. The mean CSF penetration of sorafenib was 0.02% and 3.4% after correcting for plasma protein binding. Conclusion Sorafenib is well tolerated in NHP and measurable in CSF after an IV dose. The CSF penetration of sorafenib is limited relative to total and free drug exposure in plasma.

Similar content being viewed by others
References
Wilhelm SM et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Lowy DR, Willumsen BM (1993) Function and regulation of ras. Annu Rev Biochem 62:851–891
Rodenhuis S (1992) Ras and human tumors. Semin Cancer Biol 3(4):241–247
Satoh T, Kaziro Y (1992) Ras in signal transduction. Semin Cancer Biol 3(4):169–177
Reuter CW, Morgan MA, Bergmann L (2000) Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies? Blood 96(5):1655–1669
Mercer KE, Pritchard CA (2003) Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta 1653(1):25–40
Minniti G et al (2009) Chemotherapy for glioblastoma: current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res 29(12):5171–5184
Newton HB (2007) Small-molecule and antibody approaches to molecular chemotherapy of primary brain tumors. Curr Opin Investig Drugs 8(12):1009–1021
Forshew T et al (2009) Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol 218(2):172–181
Tatevossian RG et al (2010) MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J Cell Physiol 222(3):509–514
Jain RK et al (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8(8):610–622
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neurooncol 50(1–2):109–120
Jane EP, Premkumar DR, Pollack IF (2006) Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther 319(3):1070–1080
Yang F et al (2008) Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther 7(11):3519–3526
McCully CL et al (1990) A rhesus monkey model for continuous infusion of drugs into cerebrospinal fluid. Lab Anim Sci 40(5):520–525
Freireich EJ et al (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 50(4):219–244
Jain L et al (2008) Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal 46(2):362–367
Administration, U.D.o.H.a.H.S.F.a.D., Guidance for Industry, Bioanalytical Method Validation. May 2001
Healthcare B. Nexavar (sorafenib) package insert. 2005: West Haven, CT
Strumberg D et al (2007) Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12(4):426–437
Widemann B et al (2009) Phase I study of sorafenib in children with refractory solid tumors: a children’s oncology group phase I consortium trial. J Clin Oncol 27(15s): p. Suppl; abstr 10012
Strumberg D et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Awada A et al (2005) Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 92(10):1855–1861
Moore M et al (2005) Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol 16(10):1688–1694
Clark JW et al (2005) Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 11(15):5472–5480
Jacobs S et al. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol 65(5): 817–824
Fox E et al (2002) Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther 301(3):1003–1011
Valcamonico F et al (2009) Long-lasting successful cerebral response with sorafenib in advanced renal cell carcinoma. J Neurooncol 91(1):47–50
Massard C et al (2010) Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol 21(5):1247–1254
Escudier B et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The views expressed do not necessarily represent the views of the National Institutes of Health or the US government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, A., McCully, C., Cruz, R. et al. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non-human primates. Invest New Drugs 30, 524–528 (2012). https://doi.org/10.1007/s10637-010-9585-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9585-1