Summary
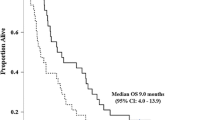
Gemcitabine is widely used for the treatment of advanced biliary tract cancer (BTC) as first-line chemotherapy. However, there is no standard chemotherapy for patient with advanced BTC refractory to gemcitabine. We conducted a multicenter phase II study of S-1 monotherapy as second-line chemotherapy for patients with advanced BTC that were refractory to gemcitabine. S-1 was administered orally at a dose of 80 mg/m2 for 28 days, followed by 14 days of rest. This regimen was repeated every 6 weeks. Tumor response was assessed every two cycles using the Response Evaluation Criteria in Solid Tumors version 1.0. Twenty-two patients were enrolled between March 2007 and January 2010, with 14 patients (64%) representing cases of recurrence after surgery. The overall response rate was 22.7%, and the overall disease control rate was 50.0%. The median overall survival time was 13.5 months (95% CI, 7.1–23.1 months) and the median time-to-progression was 5.4 months (95% CI, 2.6–17.2 months). Grade 3/4 toxicities included neutropenia (5%) and anemia (5%). The most common non-hematological toxicities were nausea (27%), anorexia (55%), and pigmentation (32%). In conclusion, S-1 monotherapy is feasible and moderately efficacious second-line chemotherapy for advanced BTC.


Similar content being viewed by others
References
National Cancer Center. Cancer statistics in Japan 2009. http://www.fpcr.or.jp/publication/statistics.html. Accessed 1 August, 2010.
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96(6):896–902
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E et al (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23(10):2332–2338
Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O et al (2008) Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 99(6):862–867
Sasaki T, Isayama H, Nakai Y, Ito Y, Kogure H, Togawa O et al (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 65(6):1101–1107
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A et al (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103(4):469–474
Lee MA, Woo IS, Kang JH, Hong YS, Lee KS (2004) Gemcitabine and cisplatin combination chemotherapy in intrahepatic cholangiocarcinoma as second-line treatment: report of four cases. Jpn J Clin Oncol 34(9):547–550
Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C et al (2007) Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 72(1–2):105–110
Oh SY, Jeong CY, Hong SC, Kim TH, Ha CY, Kim HJ, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2010 (in press).
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H et al (2010) Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs (in press).
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 91(10):1769–1774
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62(5):849–855
Park I, Lee JL, Ryu MH, Kim TW, Chang HM, Lee SS et al (2009) Efficacy and safety of S-1 monotherapy in patients with advanced biliary tract adenocarcinoma: retrospective analysis of 162 patients. Oncology 76(2):126–132
Sasaki T, Isayama H, Yashima Y, Yagioka H, Kogure H, Arizumi T et al (2009) S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 77(1):71–74
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38(1):143–151
Suzuki E, Ikeda M, Okusaka T, Nakamori S, Ohkawa S, Nagakawa T et al (2010) A multicenter phase II of S-1 in gemcitabine-refractory biliary tract cancer. Proc Am Soc Clin Oncol 28:15s, abstract no: 4145
Yonemoto N, Furuse J, Okusaka T, Yamao Funakoshi A, Ohkawa S et al (2007) A multi-centerretrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37(11):843–851
Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Ito Y et al (2010) Prognostic factors in patientswith advanced biliary tract cancer receiving chemotherapy. Cancer Chemother Pharmacol (in press)
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasaki, T., Isayama, H., Nakai, Y. et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 30, 708–713 (2012). https://doi.org/10.1007/s10637-010-9553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9553-9