Summary
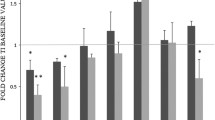
Background Bevacizumab, a monoclonal antibody (mAb) targeting vascular endothelial growth factor (VEGF), has produced promising results when combined with chemotherapy in the treatment of advanced colorectal cancer (CRC). The aim of the present study was to define the immunological profile of metastatic CRC patients at baseline and following chemotherapy with either irinotecan/5-fluorouracil/leucovorin (IFL) alone or IFL in combination with.bevacizumab (B-IFL). Methods Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors (HD) (n = 20) and patients (n = 40) were tested for T-cell proliferation in the autologous mixed lymphocyte reaction (auto-MLR), and cytokine production following stimulation with anti-CD3 mAb. Results,PBMCs obtained from CRC patients prior to treatment exhibited lower auto-MLR responses and low production of IL-2, IFN-γ, IL-12 and IL-18 cytokines, whereas IL-4 and IL-10 cytokines were increased as compared to HD (p < 0.001, for all parameters) following in vitro stimulation with anti-CD3 mAb. During treatment, and in particular in week 12 of evaluation, IL-2 (p < 0.001 for both IFL and B-IFL groups), IFN-γ (p < 0.001 for IFL and p = 0.001 for B-IFL), IL-12 (p < 0.001 for both IFL and B-IFL) and IL-18 (p < 0.001 for both IFL and B-IFL) production, as well as auto-MLR responses increased (p < 0.001 for both IFL and B-IFL), whereas IL-4 (p < 0.001 for IFL and p = 0.001 for B-IFL) and IL-10 [p < 0.001 for IFL and p = 0.067 (non-significant) for B-IFL] production decreased over baseline in the two treatment groups, yet their respective values never reached those of HD. Moreover, IL-2, IFN-γ production, and auto-MLR were higher in the B-IFL over the IFL treatment group (p < 0.001, p < 0.04, p < 0.001, respectively). Conclusion Our study demonstrates that the abnormal immune parameters observed in metastatic CRC patients at presentation can substantially improve during treatment with either IFL or B-IFL. The immune parameters examined can provide a sensitive and valuable tool for monitoring immune function in CRC patients, and could be applied as surrogate markers predicting treatment-related outcome.
Similar content being viewed by others
References
Pfeiffer P, Qvortrup C, Eriksen JG (2007) Current role of antibody therapy in patients with metastatic colorectal cancer. Oncogene 26:3661–3678
Majer M, Akerley W, Kuwada SK (2007) Oncologists’ current opinion on the treatment of colon carcinoma. Anticancer Agents Med Chem 7:492–503
Board RE, Valle JW (2007) Metastatic colorectal cancer: current systemic treatment options. Drugs 67(13):1851–1867
Wils J (2007) Adjuvant treatment of colon cancer: past, present and future. J Chemother 19:115–122
Sorscher SM (2007) Biological therapy update in colorectal cancer. Expert Opin Biol Ther 7:509–519
Giantonio BJ, Levy DE, O’Dwyer P, Meropol NJ, Catalano PJ, Benson AB 3rd (2006) A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 17:1399–1403
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Mulcahy MF, Benson AB 3rd (2005) Bevacizumab in the treatment of colorectal cancer. Expert Opin Biol Ther 5:997–1005
Berghella AM, Pellegrini P, Del Beato T, Marini M, Tomei E, Adorno D, Casciani CU (1998) The significance of an increase in soluble interleukin-2 receptor level in colorectal cancer and its biological regulating role in the physiological switching of the immune response cytokine network from TH1 to TH2 and back. Cancer Immunol Immunother 45:241–249
Shibata M, Nezu T, Takekawa M, Takizawa H, Ando K, Miyake H, Amano S, Kurosu Y (1996) Serum levels of interleukin-10 and interleukin-12 in patients with colorectal cancer. Ann N Y Acad Sci 795:410–412
Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, Imai Y, Shimomukai H, Nomura Y, Matsuda Y, Matsuzawa Y (1996) High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology 110:375–382
Zaloudik J, Lauerova L, Janakova L, Talac R, Simickova M, Nekulova M, Mikulikova I, Kovarik J, Sheard M (1999) Significance of pre-treatment immunological parameters in colorectal cancer patients with unresectable metastases to the liver. Hepatogastroenterology 46:220–227
Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG (2000) Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer 82:1009–1012
Lahm H, Schindel M, Frikart L, Cerottini JP, Yilmaz A, Givel JC, Fischer JR (1998) Selective suppression of cytokine secretion in whole blood cell cultures of patients with colorectal cancer. Br J Cancer 78:1018–1023
O’Hara RJ, Greenman J, Drew PJ, McDonald AW, Duthie GS, Lee PW, Monson JR (1998) Impaired interleukin-12 production is associated with a defective anti-tumor response in colorectal cancer. Dis Colon Rectum 41:460–463
Brew R, Southern SA, Flanagan BF, McDicken IW, Christmas SE (1996) Detection of interleukin-8 mRNA and protein in human colorectal carcinoma cells. Eur J Cancer 32A:2142–2147
Gastl GA, Abrams JS, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, Bander NH (1993) Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 55:96–101
Langerak AD, Garewal HS (1999) Transforming growth factor-beta1: a useful tumor marker in patients with colorectal carcinoma? Cancer 85:517–519
Maeurer MJ, Walter W, Martin D, Zitvogel L, Elder E, Storkus W, Lotze MT (1997) Interleukin-7 (IL-7) in colorectal cancer: IL-7 is produced by tissues from colorectal cancer and promotes preferential expansion of tumour infiltrating lymphocytes. Scand J Immunol 45:182–192
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M (2000) Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol 164:3902–3912
Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M (2002) Large-scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother 51:440–448
Tsavaris N, Baxevanis C, Kosmidis P, Papamichael M (1996) The prognostic significance of immune changes in patients with renal cancer, melanoma and colorectal cancer, treated with interferon alpha 2b. Cancer Immunol Immunother 43:94–102
Baxevanis CN, Tsiatas ML, Cacoullos NT, Spanakos G, Liacos C, Missitzis I, Papadhimitriou SI, Papamichail M (1997) Induction of anti-tumour lymphocytes in cancer patients after brief exposure to supernatants from cultures of anti-CD3-stimulated allogeneic lymphocytes. Br J Cancer 76:1072–1080
Cher DJ, Mosmann TR (1987) Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol 138:3688–3694
Li HJ, Wang QE, Yu LZ, Ding Y, Liou LB, Guo YL (2002) Detection of cell apoptosis induced with anti-Fas antibody in bladder carcinoma cell line by single cell gel electrophoresis. Ai Zheng 21:45–49
Stevens TL, Bossie A, Sanders VM et al (1988) Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255–258
Swain SL, Weinberg AD, English M, Huston G (1990) IL-4 directs the development of Th2-like helper effectors. J Immunol 145:3796–3806
Gajewski TF, Fitch FW (1988) Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol 140:4245–4252
Powrie F, Menon S, Coffman RL (1993) Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol 23:3043–3049
Gritzapis AD, Baxevanis CN, Missitzis I, Katsanou ES, Alexis MN, Yotis J, Papamichail M (2003) Quantitative fluorescence cytometric measurement of estrogen and progesterone receptors: correlation with the hormone binding assay. Breast Cancer Res Treat 80:1–13
Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M (1999) Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 31:323–331
Anastasopoulos E, Reclos GJ, Baxevanis CN, Gritzapis AD, Tsilivakos V, Panagiotopoulos N, Fotiou S, Missitzis I, Karydas I (1992) Papamichail. Monocyte disorders associated with T cell defects in patients with solid tumors. Anticancer Res 12:489–494
Nakashima H, Tasaki A, Kubo M, Kuroki H, Matsumoto K, Tanaka M, Nakamura M, Morisaki T, Katano M (2005) Effects of docetaxel on antigen presentation-related functions of human monocyte-derived dendritic cells. Cancer Chemother Pharmacol 55:479–487
Ferrari S, Malugani F, Rovati B, Porta C, Riccardi A, Danova M (2005) Flow cytometric analysis of circulating dendritic cell subsets and intracellular cytokine production in advanced breast cancer patients. Oncol Rep 14:113–120
Tormey VJ, Faul J, Leonard C, Burke CM, Dilmec A, Poulter LW (1997) T-cell cytokines may control the balance of functionally distinct macrophage populations. Immunology 90:463–469
Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Arsenis P, Katsiyiannis A, Mitsis PG, Tsavaris N, Papamichail M (1993) Comparison of immune parameters in patients with one or two primary malignant neoplasms. Nat Immun 12:41–49
Moudgil A, Toyoda M, Galfayan K, Jordan SC (1997) Selective expression of the interleukin-2 gene discriminates between the auto- and allo-mixed lymphocyte reaction. Transpl Immunol 5:35–38
Acknowledgements
The authors thank Dr. John Xynos, Department of Medicine, Royal Marsden Hospital, Sutton, Surrey, UK for his helpful discussions and help in English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsavaris, N., Voutsas, I.F., Kosmas, C. et al. Combined treatment with Bevacizumab and standard chemotherapy restores abnormal immune parameters in advanced colorectal cancer patients. Invest New Drugs 30, 395–402 (2012). https://doi.org/10.1007/s10637-010-9533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9533-0