Summary
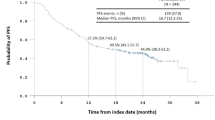
Topotecan, a semi-synthetic camptothecin analogue with topoisomerase I interaction, has shown to be an active agent in the treatment of advanced refractory lung cancer. This paper describes the authors’ experience with this drug when used as a single agent in patients (pts) with advanced non-small cell lung cancer (NSCLC) refractory to platinum- and taxane-containing chemotherapy regimens. Thirty-five patients with NSCLC refractory to previous chemotherapy and KI ≥ 60% were included in the study. Their characteristics are as follows: median age of 52 years (range 43–69) and Karnofsky PS of 70 (60–80); 27 were male and 8 were female. Twenty-one (60%) patients had adenocarcinoma; eleven (31.4%), squamous cell, and three (8.5%), undifferentiated carcinoma. There was a median of two disease sites and two prior chemotherapy regimens. Topotecan was administered at a dose of 1.25 mg/m2 I.V. daily for 5 days, repeated every 21 days until disease progression, maximal response, or intolerable toxicity. After 73 cycles, patients received a median of 2 treatment cycles (1–9). All patients except one were considered evaluable for toxicity; eight episodes (24%) of nausea/vomiting and two episodes (6%) of grade 1–2 asthenia, respectively, were reported. Four (12%) patients developed grade 1–2 anemia and two (6%) subjects suffered grade 3 anemia. Seven (21%) patients had grade 1–2 neutropenia and one (3%) presented grade 5 neutropenia. In 33 patients evaluable for activity of the 35 subjects included in the study; one (2.8%) presented a partial response; nine (25.7%) had stable disease, and 23 (65.7%) exhibited disease progression. Median time to progression and overall survival were 54 (12–210) and 70 (12–324) days, respectively. Intravenous topotecan at that dose and administration schedule displays scant activity in terms of response rate in individuals with advanced NSCLC previously treated with platinum and taxanes. The role and usefulness of chemotherapy in this setting warrants further investigation and confirmation through comparative studies.
Similar content being viewed by others
References
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18(12):2354–2362
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Yao Tsao TC, Pless M, Muller T, Lim H, Desch C, Szondy K, Gervais R, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597
Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M (1989) DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science 246(4933):1046–1048
Wall JG, Burris HA 3rd, Von Hoff DD, Rodriguez G, Kneuper-Hall R, Shaffer D, O’Rourke T, Brown T, Weiss G, Clark G (1992) A phase I clinical and pharmacokinetic study of the topoisomerase I inhibitor topotecan (SK&F 104864) given as an intravenous bolus every 21 days. Anticancer Drugs 3(4):337–345
Saltz L, Sirott M, Young C, Tong W, Niedzwiecki D, Tzy-Jyun Y, Tao Y, Trochanowski B, Wright P, Barbosa K, Toomasi F, Kelsen D (1993) Phase I clinical and pharmacology study of topotecan given daily for 5 consecutive days to patients with advanced solid tumors, with attempt at dose intensification using recombinant granulocyte colony-stimulating factor. J Natl Cancer Inst 85(18):1499–1507
Rowinsky EK, Grochow LB, Hendricks CB, Ettinger DS, Forastiere AA, Hurowitz LA, McGuire WP, Sartorius SE, Lubejko BG, Kaufmann SH (1992) Phase I and pharmacologic study of topotecan: a novel topoisomerase I inhibitor. J Clin Oncol 10(4):647–656
Verweij J, Lund B, Beijnen J, Planting A, de Boer-Dennert M, Koier I, Rosing H, Hansen H (1993) Phase I and pharmacokinetics study of topotecan, a new topoisomerase I inhibitor. Ann Oncol 4(8):673–678
Markman M (1997) Topotecan: an important new drug in the management of ovarian cancer. Semin Oncol 4(Suppl 5):55–58
Ardizzoni A, Hansen H, Dombernowsky P, Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J, Verweij J (1997) Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15(5):2090–2096
Greco FA (2003) Topotecan as first-line therapy for small cell lung cancer. Lung Cancer 41(Suppl 4):S9–S16
Jensen PB, Holm B, Sorensen M, Christensen IJ, Sehested M (1997) In vitro cross-resistance and collateral sensitivity in seven resistant small-cell lung cancer cell lines: preclinical identification of suitable drug partners to taxotere, taxol, topotecan and gemcitabine. Br J Cancer 75(6):869–877
Gervais R, Quoix E, Breton JL, Mattson K, Wilson J, Ross G (2001) Randomized phase II study of topotecan/cisplatin (TC) versus topotecan/etoposide (TE) in patients with untreated, extensive disease, small-cell lung cancer. Proc Am Soc Clin Oncol 20:318a
Lynch TI, Kalish L, Strauss G, Elias A, Skarin A, Shulman LN, Posner M, Frei E (1994) Phase II study of topotecan in metastatic non-small-cell lung cancer. J Clin Oncol 12:347–352
Perez-Soler R, Fossella FV, Glisson BS, Lee JS, Murphy WK, Shin DM, Kemp BL, Lee JJ, Kane J, Robinson RA, Lippman SM, Kurie JM, Huber MH, Raber MN, Hong WK (1996) Phase II study of topotecan in patients with advanced non-small-cell lung cancer previously untreated with chemotherapy. J Clin Oncol 14:503–513
Perez-Soler R, Khuri F, Pisters KM, Robinson R, Wimberly A, Lee JK, Fossella FV (1997) Phase II study of topotecan in patients with squamous carcinoma of the lung previously untreated with chemotherapy. Proc Am Soc Clin Oncol 16:450a
Kindler HL, Kris MG, Smith IE, Miller VA, Grant SC, Krebs JB, Ross GA, Slevin ML (1998) Phase II trial of topotecan administered as a 21-day continuous infusion in previously untreated patients with stage IIIB and IV non-small-cell lung cancer. Am J Clin Oncol 21:438–441
Mainwaring PN, Nicolson MC, Hickish T, Penson R, Joel S, Slevin M, Smith IE (1997) Continuous infusional topotecan in advanced breast and non-small-cell lung cancer: no evidence of increased efficacy. Br J Cancer 76(12):1636–1639
Weitz JJ, Marschke RF, Sloan JA, Grill JP, Jett JR, Knost JA, Hatfield AK, Zenk DW, Bate WW, Schaefer PL (2000) A randomized phase II trial of two schedules of topotecan for the treatment of advanced stage non-small cell lung cancer. Lung Cancer 28(2):157–162
Gerrits CJH, Burris H, Schellens JHM, Planting AST, van den Burg MEL, Rodriguez GI, van Beurden V, Loos WJ, Hudson I, Fields S, Verweij J, von Hoff DD (1998) Five days of oral topotecan (hycamtin®), a phase I and pharmacological study in adult patients with solid tumours. Eur J Cancer 34(7):1030–1035
White SC, Cheeseman S, Thatcher N, Anderson H, Carrington B, Hearn S, Ross G, Ranson M (2000) Phase II study of oral topotecan in advanced non-small cell lung cancer. Clin Cancer Res 6(3):868–873
Rowinsky EK, Grochow LB, Hendricks CB, Ettinger DS, Forastiere AA, Hurowitz LA, McGuire WP, Sartorius SE, Lubejko BG, Kaufmann SH (1992) Phase I and pharmacologic study of topotecan: a novel topoisomerase I inhibitor. J Clin Oncol 10(4):647–656
Hochster H, Liebes L, Speyer J, Sorich J, Taubes B, Oratz R, Wernz J, Chachoua A, Raphael B, Vinci RZ (1994) Phase I trial of low dose continuous topotecan infusion in patients with cancer: an active and well tolerated regimen. J Clin Oncol 12(3):553–559
World Health Organization (1979) Handbook for reporting results of cancer treatment. WHO offset publication no. 48, Geneva
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457–481
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Ramlau R, Gervais R, Krzakowski M, Van Pawel J, Kaukel E, Abratt RP, Dharan B, Grotzinger KM, Ross G, Shepherd FA (2006) Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol 24(18):2800–2807
Massarelli E, Andre F, Liu DD, Lee JJ, Wolf M, Fandi A, Ochs J, Le Chevalier T, Fossella F, Herbst RS (2003) A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer 39(1):55–61
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med 353(2):123–132
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES et al (2009) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372(9652):1809–1818
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez, E.E., Villanueva, N., Fra, J. et al. Activity of topotecan given intravenously for 5 days every three weeks in patients with advanced non-small cell lung cancer pretreated with platinum and taxanes: a phase II study. Invest New Drugs 29, 1459–1464 (2011). https://doi.org/10.1007/s10637-010-9442-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9442-2