Summary
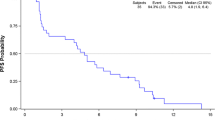
Aim Phase II multi-disease randomized discontinuation trial to assess the safety and efficacy of sorafenib including patients with advanced soft tissue sarcoma (STS). Methods Sorafenib (400 mg twice daily) was initially administered for 12 weeks. Patients with: ≥25% tumour shrinkage continued sorafenib; ≥25% tumour growth discontinued; other patients were randomized and received sorafenib or placebo. Results Twenty-six patients (median age 55 years) were enrolled. Common drug-related adverse events, including fatigue, hand–foot skin reaction, rash or gastrointestinal disturbances, were manageable, reversible and generally low grade. Fatigue, skin toxicity, nausea, diarrhoea and hypertension occurred at grade ≥3 in 19% of patients. After 12 weeks eight (31%) patients had not progressed. Three patients who experienced tumour shrinkage and continued on sorafenib, and five (19%) were randomized either to continue sorafenib or to receive placebo. Of the three patients randomized to sorafenib, one achieved a partial response and two had SD. Overall one patient achieved a partial response and three further patients achieved minor responses. Conclusions There was evidence of disease activity in STS as defined by tumor regressions including one objective partial response. Further investigation in STS is warranted.


Similar content being viewed by others
References
George S (2007) Sunitinib, a multitargeted tyrosine kinase inhibitor, in the management of gastrointestinal stromal tumor. Curr Oncol Rep 9(4):323–327
Siehl J, Thiel E (2007) C-kit, GIST, and imatinib. Recent Results Cancer Res 176:145–151
Van GM, van Oosterom AT, Oosterhuis JW et al (1999) Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2, 185 patients treated with anthracycline-containing first-line regimens–a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17(1):150–157
Nielsen OS, Judson I (2000) van HQ, et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 36(1):61–67
Nielsen OS, Dombernowsky P, Mouridsen H et al (1998) High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78(12):1634–1639
Judson I, Radford JA, Harris M et al (2001) Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 37(7):870–877
Benjamin RS, Rouesse J, Bourgeois H, van Hoesel QG (1998) Should patients with advanced sarcomas be treated with chemotherapy? Eur J Cancer 34(7):958–965
Penel N, Nisse C, Feddal S, Lartigau E (2001) Epidemiology of soft tissue sarcomas in adults. Presse Med 30(28):1405–1413
Penel N, Nisse C, Feddal S, Lartigau E (2001) Epidemiology of soft tissue sarcomas in adults. Presse Med 30(28):1405–1413
Verweij J, Casali PG, Zalcberg J et al (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364(9440):1127–1134
Talpaz M, Shah NP, Kantarjian H et al (2006) Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354(24):2531–2541
Demetri GD, van Oosterom AT, Garrett CR et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368(9544):1329–1338
Kasper B, Gil T, D’Hondt V, Gebhart M, Awada A (2007) Novel treatment strategies for soft tissue sarcoma. Crit Rev Oncol Hematol 62(1):9–15
Wilhelm S, Chien DS (2002) BAY 43–9006: preclinical data. Curr Pharm Des 8(25):2255–2257
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Ratain MJ, Eisen T, Stadler WM et al (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Ratain MJ, Eisen T, Stadler WM et al (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512
Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49(17):4682–4689
Davies H, Bignell GR, Cox C et al (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954
Ratain MJ, Eisen T, Stadler WM et al (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512
Ratain MJ, Eisen T, Stadler WM et al (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47(1):207–214
Maki RG, D’Adamo DR, Keohan ML et al (2009) Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol May 18
Ryan CW, von Mehren M, Rankin CJ et al (2008) Phase II intergroup study of sorafenib (S) in advanced soft tissue sarcomas (STS): SWOG 0505. J Clin Oncol 26:10532
Wiebe L, Kasra KE, Maki RG et al (2008) Activity of sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol 26(suppl):10502
Reichardt P, Montemurro M, Gelderbloom H et al (2009) Sorafenib fourth-line treatment in imatinib-, sunitinib-, and nilotinib-resistant metastatic GIST: a retrospective analysis. J Clin Oncol 27(15s):10564
Tap WD, Federman N, Eilber FC (2007) Targeted therapies for soft-tissue sarcomas. Expert Rev Anticancer Ther 7(5):725–733
Kasper B, Gil T, D’Hondt V, Gebhart M, Awada A (2007) Novel treatment strategies for soft tissue sarcoma. Crit Rev Oncol Hematol 62(1):9–15
Sleifer S, Papai Z, Le CA et al (2007) Phase II study of pazopanib (GW786034) in patients (pts) with relapsed or refractory soft tissue sarcoma (STS): EORTC 62043. J Clin Oncol 25(18S):10031
Heymach JV, Desai J, Manola J et al (2004) Phase II study of the antiangiogenic agent SU5416 in patients with advanced soft tissue sarcomas. Clin Cancer Res 10(17):5732–5740
Guida T, Anaganti S, Provitera L et al (2007) Sorafenib Inhibits Imatinib-Resistant KIT and Platelet-Derived Growth Factor Receptor beta Gatekeeper Mutants. Clin Cancer Res 13(11):3363–3369
Acknowledgements
Assistance in drafting this manuscript was provided by Chameleon and paid for by Bayer Pharmaceuticals Ltd. Bayer held the database, provided statistical support and provided information on toxicity and response. Bayer was not involved in the decision to publish the data on sarcoma or in the interpretation of the data. Dr A Patnaik assisted with patient recruitment and treatment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacey, S., Ratain, M.J., Flaherty, K.T. et al. Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs 29, 481–488 (2011). https://doi.org/10.1007/s10637-009-9367-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9367-9