Summary
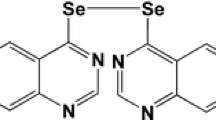
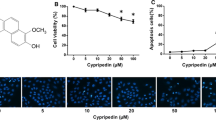
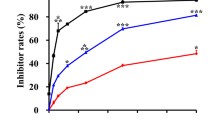
In our previous study, a series of novel cyclic cyanoguanidine compounds, eg. 5-substituted 2-cyanoimino-4-imidazodinone and 2-cyanoimino-4- pyrimidinone derivatives have been successfully synthesized and showed remarkable cytotoxicity in several cancer cell lines. In this present study, it is our aim to screen more potential candidates among the cyclic pyridyl cyanoguanidine compounds (BPR-DC-1, 2, 3) by in vitro and in vivo studies for the therapy of lung cancer, alternatively. Our results showed that BPR-DC-2 significantly inhibited proliferation of tumor cells with an IC50 of 3.60 ± 1.27 and 14.81 ± 4.23 μM in human lung carcinoma cells, H69 and A549, respectively by the MTT assay at 48 hr; BPR-DC-2 also obviously suppressed the tumor proliferation and MDR-1 gene expression, even induced cell apoptosis in the ex vivo histocultured lung tumor. We further demonstrated that, in the nude mouse model of metastatic lung cancer, BPR-DC-2 could diminish the tumor mass, retard the progression of metastasis, and prolong the survival time. In addition, it was found that BPR-DC-2 exerted its anti-tumor effects through the inhibition of MDR-1 gene expression and down-regulation of tumor anti-apoptosis signals (activated p-AKT and over-expression of PARP-1) by western blotting analysis. In conclusion, in this present study we have demonstrated that BPR-DC-2, derived from a series of novel synthetic cyclic cyanoguanidine compounds, has proved its potential as an anti-tumor drug candidate in treating lung cancer.






Similar content being viewed by others
References
Liu CC, Tsai SS, Chiu HF, Wu TN, Yang CY (2008) Ambient exposure to criteria air pollutants and female lung cancer in Taiwan. Inhal. Toxicol. 20:311–317
Cheng YW, Chiou HL, Sheu GT, Hsieh LL, Chen JT, Chen CY, Su JM, Lee H (2001) The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res 61:2799–2803
Chen CJ, Wu HY, Chuang YC, Chang AS, Luh KT, Chao HH, Chen KY, Chen SG, Lai GM, Huang HH, Lee HH (1990) Epidemiologic characteristics and multiple risk factors of lung cancer in Taiwan. Anticancer Res 10:971–976
Ramalingama S, Belanib C (2008) Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. The Oncologist 13(suppl 1):5–13
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics. CA Cancer J Clin 58:71–96
Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR (1996) Improved Survival in Stage III Non-Small-Cell Lung Cancer: Seven-Year Follow-up of Cancer and Leukemia Group B (CALGB) 8433 Trial. J Natl Cancer Inst 88:1210–1215
Younes MN, Park YW, Yazici YD, Gu M, Santillan AA, Nong X, Kim S, Jasser SA, El-Naggar AK, Myers JN (2006) Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary adenoid cystic carcinoma in an orthotopic nude mouse model. Mol Cancer Ther 5:2696–2705
de Haan LD, De Mulder PH, Vermorken JB, Schornagel JH, Vermey A, Verweij J (1992) Cisplatin-based chemotherapy in advanced adenoid cystic carcinoma of the head and neck. Head Neck 14:273–277
Olsen LS, Hjarnaa PJ, Latini S, Holm PK, Larsson R, Bramm E, Binderup L, Madsen MW (2004) Anticancer agent CHS 828 suppresses nuclear factor-kappa B activity in cancer cells through downregulation of IKK activity. Int J Cancer 111:198–205
Mader MM (2005) Novel antiproliferative antitumor agents. Curr Opin Drug Discov Devel 8:613–618
Hassan SB, Jonsson E, Larsson R, Karlsson MO (2001) Model for time dependency of cytotoxic effect of CHS 828 in vitro suggests two different mechanisms of action. J Pharmacol Exp Ther 299:1140–1147
Aleskog A, Bashir-Hassan S, Hovstadius P, Kristensen J, Höglund M, Tholander B, Binderup L, Larsson R, Jonsson E (2001) Activity of CHS 828 in primary cultures of human hematological and solid tumors in vitro. Anticancer Drugs 12:821–827
Singh B, Li R, Xu L, Poluri A, Patel S, Shaha AR, Pfister D, Sherman E, Goberdhan A, Hoffman RM, Shah J (2002) Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck 24:437–442
Tanino H, Oura S, Hoffman RM, Kubota T, Furukawa T, Arimoto J, Yoshimasu T, Hirai I, Bessho T, Suzuma T, Sakurai T, Naito Y (2001) Acquisition of multidrug resistance in recurrent breast cancer demonstrated by the histoculture drug response assay. Anticancer Res 21:4083–4086
Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, Morel P, Fenaux P (1994) p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood 84:3148–3157
Cavalcanti GB Jr, da Cunha Vasconcelos F, Pinto de Faria G, Scheiner MA, de Almeida Dobbin J, Klumb CE, Maia RC (2004) Coexpression of p53 protein and MDR functional phenotype in leukemias: the predominant association in chronic myeloid leukemia. Cytometry B Clin Cytom 61:1–8
Lee CH, Wu CL, Shiau AL (2007) Hypoxia-induced cytosine deaminase gene expression for cancer therapy. Hum Gene Ther 18:27–38
Han JY, Chung YJ, Park SW, Kim JS, Rhyu MG, Kim HK, Lee KS (1999) The relationship between cisplatin-induced apoptosis and p53, bcl-2 and bax expression in human lung cancer cells. Korean J Intern Med 14:42–52
Miao ZH, Tong LJ, Zhang JS, Han JX, Ding J (2004) Characterization of salvicine-resistant lung adenocarcinoma A549/SAL cell line. Int J Cancer 110:627–632
Chuu JJ, Liu JM, Tsou MH, Huang CL, Chen CP, Wang HS, Chen CT (2007) Effects of paclitaxel and doxorubicin in histocultures of hepatocelular carcinomas. J Biomed Sci 14:233–244
Chen CT, Gan Y, Au JL, and Wientjes MG (1998) Androgen-dependent and — independent human prostate xenograft tumors as models for drug activity evaluation. Cancer Res 58:2777–2783
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Al-Ejeh F, Darby JM, Brown MP (2009) Chemotherapy synergizes with radioimmunotherapy targeting la autoantigen in Tumors. PLoS ONE 4(2):e4630. doi:10.1371/journal.pone.0004630
Sun SY, Zhou Z, Wang R, Fu H, Khuri FR (2004) The farnesyltransferase inhibitor Lonafarnib induces growth arrest or apoptosis of human lung cancer cells without downregulation of Akt. Cancer Biol Ther 3:1092–8
David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, Brody AR (2004) Phospho-Akt Overexpression in Non—Small Cell Lung Cancer Confers Significant Stage-Independent Survival Disadvantage. Clin. Cancer Res. 10:6865–6871
Leprêtre C, Scovassi AI, Shah GM, Torriglia A (2009) Regulation of poly(ADP-ribose) polymerase-1 functions by leukocyte elastase inhibitor/LEI-derived DNase II during caspase-independent apoptosis. Int J Biochem Cell Biol. 41:1046–1054
Kobayashi K, Nakanishi H, Masuda A, Tezuka N, Mutai M, Tatematsu M (1997) Sequential observation of micrometastasis formation by bacterial lacZ gene- tagged Lewis lung carcinoma cells. Cancer Lett 112:191–198
Olsen LS, Hjarnaa PJ, Latini S, Holm PK, Larsson R, Bramm E, Binderup L, Madsen MW (2004) Anticancer agent CHS 828 suppresses nuclear factor-kappa B activity in cancer cells through downregulation of IKK activity. Int J Cancer 111:198–205
Hassan SB, Lövborg H, Lindhagen E, Karlsson MO, Larsson R (2006) CHS 828 kill tumour cells by inhibiting the nuclear factor-kappaB translocation but unlikely through down-regulation of proteasome. Anticancer Res 26:4431–4436
Hjarnaa PJ, Jonsson E, Latini S, Dhar S, Larsson R, Bramm E, Skov T, Binderup L (1999) CHS 828, a novel pyridyl cyanoguanidine with potent antitumor activity in vitro and in vivo. Cancer Res 59:5751–5757
Ravaud A, Cerny T, Terret C, Wanders J, Bui BN, Hess D, Droz JP, Fumoleau P, Twelves C (2005) Phase I study and pharmacokinetic of CHS-828, a guanidino— containing compound, administered orally as a single dose every 3 weeks in solid tumours: An ECSG/EORTC study. Eur J Cancer 41:702–707
Hovstadius P, Lindhagen E, Hassan S, Nilsson K, Jernberg-Wiklund H, Nygren P, Binderup L, Larsson R (2004) Cytotoxic effect in vivo and in vitro of CHS 828 on human myeloma cell lines. Anticancer Drugs 15:63–70
Chern JH, Shia KH, Chang CM, Lee CC, Lee YC, Tai CL, Lin YT, Chang CH, Tseng HY (2004) Synthesis and in vitro cytotoxicity of 5-substituted 2- cyanoimino -4- imidazodinone and 2- cyanoimino-4-pyrimidinone derivatives. Bioorg Med Chem Lett 14:1169–172
Chuu JJ, Liu JM, Tsou MH, Huang CL, Chen CP, Wang HS, Chen CT (2007) Effects of paclitaxel and doxorubicin in histocultures of hepatocelular carcinomas. J Biomed Sci 14:233–244
Chang SG, Jung JC, Rho YS, Huh JS, Kim JI, Hoffman RM (1996) Efficacy of the platinum analog [Pt(cis-dach)(DPPE)-2NO3] on histocultured human patient bladder tumors and cancer cell lines. Anticancer Res 16:3423–3428
Jang SH, Wientjes MG, Au JL (2001) Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Investi New Drugs 19:113–123
Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL (1999) Determinants of paclitaxel penetration and accumulation in human solid tumor. J. Pharmacol. Exp. Ther. 290:871–880
Au JL, Li D, Gan Y, Gao X, Johnson AL, Johnston J, Millenbaugh NJ, Jang SH, Kuh HJ, Chen CT, Wientjes MG (1998) Pharmacodynamics of immediate and delayed effects of paclitaxel: role of slow apoptosis and intracellular drug retention. Cancer Res 58:2141–2148
Zheng JH, Chen CT, Au JL, Wientjes MG (2001) Time-and concentration- dependent penetration of doxorubicin in prostate tumors. Aaps Pharmsci 3:E15
Blumenthal RD, Osorio L, Hayes MK, Horak ID, Hansen HJ, Goldenberg DM (2005) Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol Immunother 54:315–327
Thompson JA, Eades-Perner AM, Ditter M, Muller WJ, Zimmermann W (1997) Expression of transgenic carcinoembryonic antigen (CEA) in tumor- rone mice: an animal model for CEA-directed tumor immunotherapy. Int J Cancer 72:197–202
Furukawa T, Kubota T, Murata H, Tanino H, Yuasa S, Morita K, Ueno J, Kozakai K, Yano T (1998) Antitumor spectra of anthracyclines against gastric cancer tissues obtained from surgical specimens with reference to P-glycoprotein expression. J Surg Oncol 69:173–177
Gupta AK, Soto DE, Feldman MD, Goldsmith JD, Mick R, Hahn SM, Machtay M, Muschel RJ, McKenna WG (2004) Signaling pathways in NSCLC as a predictor of outcome and response to therapy. Lung 182:151–162
Shah A, Swain WA, Richardson D, Edwards J, Stewart DJ, Richardson CM, Swinson DE, Patel D, Jones JL, O'Byrne KJ (2005) Phospho-akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res 11:2930–2936
Tang JM, He QY, Guo RX, Chang XJ (2006) Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer 51:181–191
Cicenas J (2008) The potential role of Akt phosphorylation in human cancers. Int J Biol Markers 23:1–9
Shtilbans V, Wu M, Burstein DE (2008) Current overview of the role of Akt in cancer studies via applied immunohistochemistry. Ann Diagn Pathol 12:153–160
Miao LJ, Wang J, Li SS, Wu YM, Wu YJ, Wang XC (2006) Correlation of P27 expression and localization to phosphorylated AKT in non-small cell lung cancer. Ai Zheng 25:1216–1220
Lövborg H, Martinsson P, Gullbo J, Ekelund S, Nygren P, Larsson R (2002) Modulation of pyridyl cyanoguanidine (CHS 828) induced cytotoxicity by 3- minobenzamide in U-937 GTB cells. Biochem Pharmacol 63:1491–1498
Acknowledgments
The investigation was partially supported by a research grant (CCMP96-RD-015) Committee on Chinese pharmacy, department of health, executive Yuan, Taipei, Taiwan. Meanwhile, and by the National Science council, the Republic of China, under grant (NSC 94-2320-B-218 -001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, SL., Huang, CH., Lin, CC. et al. Antitumor effect of BPR-DC-2, a novel synthetic cyclic cyanoguanidine derivative, involving the inhibition of MDR-1 expression and down-regulation of p-AKT and PARP-1 in lung cancer. Invest New Drugs 29, 195–206 (2011). https://doi.org/10.1007/s10637-009-9337-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9337-2