Summary
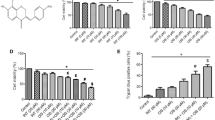
In this study, we characterized the effects of LA-12 on tumor cell lines possessing wild type p53 and on p53-deficient/mutant cell lines and the results were compared to those obtained using cisplatin. We have determined changes of p53 levels, of its transcriptional activity, of its posttranscriptional modifications and the effect of the treatment on the cell cycle, on the induction of apoptosis and on gene expression. LA-12 induces weak accumulation of both transcriptionally active p53 tumor suppressor and of p21WAF1/CIP1 protein. LA-12 and cisplatin also significantly differ in their effects on apoptosis and cell cycle and on gene expression spectra in studied cell lines. LA-12 induces higher apoptosis levels in comparison with those induced by cisplatin, especially in p53-deficient H1299 cells and in MCF-7DD cells with transcriptionally inactive p53. We suggest that LA-12-mediated apoptosis is not fully dependent on p53. This confirms the therapeutic potential of LA-12 as a more potent cytostatic agent for both tumor cells expressing wild type p53 and for p53-deficient or mutant cells.







Similar content being viewed by others
References
Adams M, A'Hern RP, Calvert AH, Carmichael J, Clark PI, Coleman RE, Earl HM, Gallagher CJ, Ganesan TS, Gore ME, Graham JD, Harper PG, Jayson GC, Kaye SB, Ledermann JA, Osborne RJ, Perren TJ, Poole CJ, Radford JA, Rustin GJ, Slevin ML, Smyth JF, Thomas H, Wilkinson PM (1998) Chemotherapy for ovarian cancer–a consensus statement on standard practice. Br J Cancer 78:1404–1406
Zlatanova J, Yaneva J, Leuba SH (1998) Proteins that specifically recognize cisplatin-damaged DNA: a clue to anticancer activity of cisplatin. FASEB J 12:791–799
Boudny V, Vrana O, Gaucheron F, Kleinwachter V, Leng M, Brabec V (1992) Biophysical analysis of DNA modified by 1, 2-diaminocyclohexane platinum(II) complexes. Nucleic Acids Res 20:267–272. doi:10.1093/nar/20.2.267
Eastman A (1999) The mechanism of action of cisplatin: from adducts to apoptosis. In: Lippert B (ed) Cisplatin, chemistry, and biochemistry of a leading anticancer drug, 1st edn. Helvetica Chimica Acta, Zürich, pp 111–135
Zak F, Turanek J, Kroutil A, Sova P, Mistr A, Poulova A, Mikolin P, Zak Z, Kasna A, Zaluska D, Neca J, Sindlerova L, Kozubik A (2004) Platinum(IV) complex with adamantylamine as nonleaving amine group: synthesis, characterization, and in vitro antitumor activity against a panel of cisplatin-resistant cancer cell lines. J Med Chem 47:761–763. doi:10.1021/jm030858+
Sova P, Chladek J, Zak F, Mistr A, Kroutil A, Semerad M, Slovak Z (2005) Pharmacokinetics and tissue distribution of platinum in rats following single and multiple oral doses of LA-12. Int J Pharm 288:123–129 (OC-6-43)-bis(acetato)(1-adamantylamine)amminedichloroplatinum(IV). doi:10.1016/j.ijpharm.2004.09.020
Kozubik A, Horvath V, Svihalkova-Sindlerova L, Soucek K, Hofmanova J, Sova P, Kroutil A, Zak F, Mistr A, Turanek J (2005) High effectiveness of platinum(IV) complex with adamantylamine in overcoming resistance to cisplatin and suppressing proliferation of ovarian cancer cells in vitro. Biochem Pharmacol 69:373–383. doi:10.1016/j.bcp.2004.09.005
Ko LJ, Prives C (1996) p53: puzzle and paradigm. Genes Dev 10:1054–1072. doi:10.1101/gad.10.9.1054
Giaccia AJ, Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev 12:2973–2983. doi:10.1101/gad.12.19.2973
Jayaraman L, Prives C (1999) Covalent and noncovalent modifiers of the p53 protein. Cell Mol Life Sci 55:76–87. doi:10.1007/s000180050271
Meek DW (1998) New developments in the multi-site phosphorylation and integration of stress signalling at p53. Int J Radiat Biol 74:729–737. doi:10.1080/095530098141005
Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E (1998) DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev 12:2831–2841. doi:10.1101/gad.12.18.2831
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. doi:10.1016/0092-8674(93)90500-P
El-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y et al (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54:1169–1174
Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76:1013–1023. doi:10.1016/0092-8674(94) 90379-4
Loignon M, Fetni R, Gordon AJ, Drobetsky EA (1997) A p53-independent pathway for induction of p21waf1cip1 and concomitant G1 arrest in UV-irradiated human skin fibroblasts. Cancer Res 57:3390–3394
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88:323–331. doi:10.1016/S0092-8674(00) 81871-1
Lane DP (1994) p53 and human cancers. Br Med Bull 50:582–599
Blaydes JP, Hupp TR (1998) DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site. Oncogene 17:1045–1052. doi:10.1038/sj.onc.1202014
Janicke RU, Sprengart ML, Wati MR, Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360. doi:10.1074/jbc.273.16.9357
Giaccone G, Battey J, Gazdar AF, Oie H, Draoui M, Moody TW (1992) Neuromedin B is present in lung cancer cell lines. Cancer Res 52:2732s–2736s
Shaulian E, Haviv I, Shaul Y, Oren M (1995) Transcriptional repression by the C-terminal domain of p53. Oncogene 10:671–680
Vojtesek B, Bartek J, Midgley CA, Lane DP (1992) An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods 151:237–244. doi:10.1016/0022-1759(92)90122-A
Stephen CW, Helminen P, Lane DP (1995) Characterisation of epitopes on human p53 using phage-displayed peptide libraries: insights into antibody-peptide interactions. J Mol Biol 248:58–78. doi:10.1006/jmbi.1995.0202
Fredersdorf S, Milne AW, Hall PA, Lu X (1996) Characterization of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol 148:825–835
Sheard MA, Krammer PH, Zaloudik J (1999) Fractionated gamma-irradiation renders tumour cells more responsive to apoptotic signals through CD95. Br J Cancer 80:1689–1696. doi:10.1038/sj.bjc.6690585
Sheard MA, Vojtesek B, Janakova L, Kovarik J, Zaloudik J (1997) Up-regulation of Fas (CD95) in human p53wild-type cancer cells treated with ionizing radiation. Int J Cancer 73:757–762. doi:10.1002/(SICI)1097-0215(19971127)73:5<757::AID-IJC24>3.0.CO;2-1
Brabec V (2002) DNA modifications by antitumor platinum and ruthenium compounds: their recognition and repair. Prog Nucleic Acid Res Mol Biol 71:1–68. doi:10.1016/S0079-6603(02)71040-4
Hrstka R, Powell DJ, Kvardova V, Roubalova E, Bourougaa K, Candeias MM, Sova P, Zak F, Fahraeus R, Vojtesek B (2008) The novel platinum(IV) complex LA-12 induces p53 and p53/47 responses that differ from the related drug, cisplatin. Anticancer Drugs 19:369–379. doi:10.1097/CAD.0b013e3282f7f500
Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4:793–805. doi:10.1038/nrc1455
Jackel M, Kopf-Maier P (1991) Influence of cisplatin on cell-cycle progression in xenografted human head and neck carcinomas. Cancer Chemother Pharmacol 27:464–471. doi:10.1007/BF00685161
Horvath V, Soucek K, Svihalkova-Sindlerova L, Vondracek J, Blanarova O, Hofmanova J, Sova P, Kozubik A (2007) Different cell cycle modulation following treatment of human ovarian carcinoma cells with a new platinum(IV) complex vs cisplatin. Invest New Drugs 5:435–443. doi:10.1007/s10637-007-9062-7
Horvath V, Blanarova O, Svihalkova-Sindlerova L, Soucek K, Hofmanova J, Sova P, Kroutil A, Fedorocko P, Kozubik A (2006) Platinum(IV) complex with adamantylamine overcomes intrinsic resistance to cisplatin in ovarian cancer cells. Gynecol Oncol 102:32–40. doi:10.1016/j.ygyno.2005.11.016
Acknowledgments
This work was supported by PLIVA-Lachema a. s. and IGA MZ CR NS/9812-4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eva Roubalová and Veronika Kvardová contributed equally to this work.
Rights and permissions
About this article
Cite this article
Roubalová, E., Kvardová, V., Hrstka, R. et al. The effect of cellular environment and p53 status on the mode of action of the platinum derivative LA-12. Invest New Drugs 28, 445–453 (2010). https://doi.org/10.1007/s10637-009-9270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9270-4