Summary
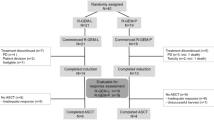
Background: Therapeutic approaches to marginal zone B-cell lymphoma (MZL) continue to evolve. Localized MZL responds favorably to local treatments, including surgery and/or local radiation therapy. However, MZL manifests as a disseminated disease in one-third of the cases at diagnosis. Moreover, relapses involving distant sites after local therapy have been reported previously. Therefore, the search for effective forms of systemic therapy is a critical issue. We conducted this multi-center, phase II trial to assess the efficacy and safety of gemcitabine single chemotherapy for patients with stage III/IV MZL. Methods: Patients received gemcitabine 1250 mg/m2 on days 1 and 8 of each cycle. The treatment was repeated every 3 weeks and continued for 6 cycles until disease progression, withdrawal due to toxicity, or withdrawal of consent. Results: Between Sep. 2006 and Sep. 2008, a total of 16 patients were enrolled (with informed consent) into this trial from 6 institutes in Korea. Among these patients, 4 patients dropped out without evaluation. The median age of the 12 (9 males, 3 females) evaluated patients was 62 (range 25–73) years. Seven patients (58%) evidenced involvement of extranodal sites. All patients received previous treatment for MZL. The patients received a total of 69 cycles of gemcitabine chemotherapy (range 3–6 [median 6] cycles/person). There were 2 PR (17%; 95% Confidence Interval [CI], 0.0–41%), 9 SD (75%), and 1 PD (8%). There were 8/69 cycles (12%) of grade 3/4 neutropenia. Non-hematologic toxicities were mild and tolerable. There were 5 cycles (8%) of delayed chemotherapy (median 1 week) owing to neutropenia. Dose reduction was required in 12 cycles. However, no treatment-related death occurred in this study. The median TTP was 10.2 months (95% CI, 5.3–15.1). As the response rate in stage I did not justify progressing to stage II (≥8/15), this study had to be discontinued, in accordance with the established protocols. Conclusion: Gemcitabine as a single agent, in this dosage and at this schedule, evidenced minimal clinical activity in cases of advanced MZL.

Similar content being viewed by others
References
Oh SY, Ryoo BY, Kim WS, Park YH, Kim K, Kim HJ, Kwon JM, Lee J, Ko YH, Ahn YC, Oh SJ, Lee SI, Kwon HC, Bang SM, Kim JH, Park J, Lee SS, Kim HY, Park K (2007) Nongastric marginal zone B-cell lymphoma: analysis of 247 cases. Am J Hematol 82:446–452. doi:10.1002/ajh.20874
Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, Salles G, Coiffier B (2000) Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 95:802–806
Zinzani PL, Magagnoli M, Galieni P, Martelli M, Poletti V, Zaja F, Molica S, Zaccaria A, Cantonetti AM, Gentilini P, Guardigni L, Gherlinzoni F, Ribersani M, Bendandi M, Albertini P, Tura S (1999) Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol 17:1254
Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, Patterson BJ, Ferreri AJ, Ponzoni M, Devizzi L, Giardini R, Pinotti G, Capella C, Zinzani PL, Pileri S, Lopez-Guillermo A, Campo E, Ambrosetti A, Baldini L, Cavalli F (2003) Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 101:2489–2495. doi:10.1182/blood-2002-04-1279
Isaacson PG (2005) Update on MALT lymphomas. Best Pract Res Clin Haematol 18:57–68. doi:10.1016/j.beha.2004.08.003
Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell’Oro S, Fleischhauer K, Caggiari L, Lettini AA, Dal Cin E, Ieri R, Freschi M, Villa E, Boiocchi M, Dolcetti R (2004) Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 96:586–594
Fung CY, Tarbell NJ, Lucarelli MJ, Goldberg SI, Linggood RM, Harris NL, Ferry JA (2003) Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes. Int J Radiat Oncol Biol Phys 57:1382–1391. doi:10.1016/S0360-3016(03)00767-3
Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Patterson BJ (2003) Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 21:4157–4164. doi:10.1200/JCO.2003.06.085
Raderer M, Streubel B, Woehrer S, Puespoek A, Jaeger U, Formanek M, Chott A (2005) High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res 11:3349–3352. doi:10.1158/1078-0432.CCR-04-2282
Hammel P, Haioun C, Chaumette MT, Gaulard P, Divine M, Reyes F, Delchier JC (1995) Efficacy of single-agent chemotherapy in low-grade B-cell mucosa-associated lymphoid tissue lymphoma with prominent gastric expression. J Clin Oncol 13:2524–2529
Jager G, Neumeister P, Brezinschek R, Hinterleitner T, Fiebiger W, Penz M, Neumann HJ, Mlineritsch B, DeSantis M, Quehenberger F, Chott A, Beham-Schmid C, Hofler G, Linkesch W, Raderer M (2002) Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase II study. J Clin Oncol 20:3872–3877. doi:10.1200/JCO.2002.05.117
Csoka K, Liliemark J, Larsson R, Nygren P (1995) Evaluation of the cytotoxic activity of gemcitabine in primary cultures of tumor cells from patients with hematologic or solid tumors. Semin Oncol 22:47–53
Dent S, Messersmith H, Trudeau M (2008) Gemcitabine in the management of metastatic breast cancer: a systematic review. Breast Cancer Res Treat 108:319–331. doi:10.1007/s10549-007-9610-z
Rivera F, Lopez-Tarruella S, Vega-Villegas MA, Salcedo M (2009) Treatment of advanced pancreatic cancer: From gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev. doi:10.1016/j.ctrv.2008.11.007
Bunn PA Jr (2002) Chemotherapy for advanced non-small-cell lung cancer: who, what, when, why? J Clin Oncol 20:23S–33S
Santoro A, Bredenfeld H, Devizzi L, Tesch H, Bonfante V, Viviani S, Fiedler F, Parra HS, Benoehr C, Pacini M, Bonadonna G, Diehl V (2000) Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. J Clin Oncol 18:2615–2619
Fossa A, Santoro A, Hiddemann W, Truemper L, Niederle N, Buksmaui S, Bonadonna G, Seeber S, Nowrousian MR (1999) Gemcitabine as a single agent in the treatment of relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol 17:3786–3792
Zinzani PL, Bendandi M, Stefoni V, Albertini P, Gherlinzoni F, Tani M, Piccaluga PP, Tura S (2000) Value of gemcitabine treatment in heavily pretreated Hodgkin’s disease patients. Haematologica 85:926–929
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored Int Working Group J Clin Oncol 17:1244
Armitage JO, Tobinai K, Hoelzer D, Rummel MJ (2004) Treatment of indolent non-Hodgkin’s lymphoma with cladribine as single-agent therapy and in combination with mitoxantrone. Int J Hematol 79:311–321. doi:10.1532/IJH97.04050
Zinzani PL, Stefoni V, Musuraca G, Tani M, Alinari L, Gabriele A, Marchi E, Pileri S, Baccarani M (2004) Fludarabine-containing chemotherapy as frontline treatment of nongastrointestinal mucosa-associated lymphoid tissue lymphoma. Cancer 100:2190–2194. doi:10.1002/cncr.20237
Raderer M, Wohrer S, Bartsch R, Prager G, Drach J, Hejna M, Gaiger A, Turetschek K, Jaeger U, Streubel B, Zielinski CC (2005) Phase II study of oxaliplatin for treatment of patients with mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 23:8442–8446. doi:10.1200/JCO.2004.00.8532
Oki Y, Younes A (2008) Current role of gemcitabine in the treatment of Hodgkin lymphoma. Leuk Lymphoma 49:883–889. doi:10.1080/10428190801911704
Pasricha SR, Grigg A, Catalano J, Leahy M, Underhill C, Arthur C, D’Rozario J, Lowenthal R, Reed K, Spencer A (2008) A multicenter phase 2 study of risk-adjusted salvage chemotherapy incorporating vinorelbine and gemcitabine for relapsed and refractory lymphoma. Cancer 113:3192–3198. doi:10.1002/cncr.23915
Corazzelli G, Russo F, Capobianco G, Marcacci G, Della Cioppa P, Pinto A (2006) Gemcitabine, ifosfamide, oxaliplatin and rituximab (R-GIFOX), a new effective cytoreductive/mobilizing salvage regimen for relapsed and refractory aggressive non-Hodgkin’s lymphoma: results of a pilot study. Ann Oncol 17(Suppl 4):iv18–iv24. doi:10.1093/annonc/mdj994
Corazzelli G, Capobianco G, Arcamone M, Ballerini PF, Iannitto E, Russo F, Frigeri F, Becchimanzi C, Marcacci G, De Chiara A, Pinto A (2009) Long-term results of gemcitabine plus oxaliplatin with and without rituximab as salvage treatment for transplant-ineligible patients with refractory/relapsing B-cell lymphoma. Cancer Chemother Pharmacol
Lopez A, Gutierrez A, Palacios A, Blancas I, Navarrete M, Morey M, Perello A, Alarcon J, Martinez J, Rodriguez J (2008) GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: a phase II study. Eur J Haematol 80:127–132
Sirohi B, Cunningham D, Norman A, Last K, Chau I, Horwich A, Oates J, Chong G, Wotherspoon A (2007) Gemcitabine, cisplatin and methylprednisolone (GEM-P) with or without Rituximab in relapsed and refractory patients with diffuse large B cell lymphoma (DLBCL). Hematology 12:149–153. doi:10.1080/10245330701214095
Crump M, Baetz T, Couban S, Belch A, Marcellus D, Howson-Jan K, Imrie K, Myers R, Adams G, Ding K, Paul N, Shepherd L, Iglesias J, Meyer R (2004) Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG). Cancer 101:1835–1842. doi:10.1002/cncr.20587
Larson BJ, Waples JM, Pusateri A, Mendenhall NP, Lynch JW Jr (2005) A phase II study of gemcitabine in patients with relapsed or refractory low-grade non-Hodgkin lymphoma. Am J Clin Oncol 28:165–168. doi:10.1097/01.coc.0000143015.66143.22
Dumontet C, Morschhauser F, Solal-Celigny P, Bouafia F, Bourgeois E, Thieblemont C, Leleu X, Hequet O, Salles G, Coiffier B (2001) Gemcitabine as a single agent in the treatment of relapsed or refractory low-grade non-Hodgkin’s lymphoma. Br J Haematol 113:772–778. doi:10.1046/j.1365-2141.2001.02795.x
Ganjoo KN, Robertson MJ, Fisher W, Jung SH, McClean J, Huh SY, Bufill J, Williams S, Cripe LD (2005) A phase II study of single agent gemcitabine in relapsed or refractory follicular or small lymphocytic non-Hodgkin lymphomas: a Hoosier Oncology Group Study. Am J Clin Oncol 28:169–172. doi:10.1097/01.coc.0000144812.74663.d0
van der Wilt CL, Kroep JR, Bergman AM, Loves WJ, Alvarez E, Talianidis I, Eriksson S, van Groeningen CJ, Pinedo HM, Peters GJ (2000) The role of deoxycytidine kinase in gemcitabine cytotoxicity. Adv Exp Med Biol 486:287–290. doi:10.1007/0-306-46843-3_56
Ruiz van Haperen VW, Veerman G, Eriksson S, Boven E, Stegmann AP, Hermsen M, Vermorken JB, Pinedo HM, Peters GJ (1994) Development and molecular characterization of a 2′, 2′-difluorodeoxycytidine-resistant variant of the human ovarian carcinoma cell line A2780. Cancer Res 54:4138–4143
Dumontet C, Fabianowska-Majewska K, Mantincic D, Callet Bauchu E, Tigaud I, Gandhi V, Lepoivre M, Peters GJ, Rolland MO, Wyczechowska D, Fang X, Gazzo S, Voorn DA, Vanier-Viornery A, MacKey J (1999) Common resistance mechanisms to deoxynucleoside analogues in variants of the human erythroleukaemic line K562. Br J Haematol 106:78–85. doi:10.1046/j.1365-2141.1999.01509.x
Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, Crawford CR, Cass CE (1998) Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res 58:4349–4357
Bergman AM, Giaccone G, van Moorsel CJ, Mauritz R, Noordhuis P, Pinedo HM, Peters GJ (2000) Cross-resistance in the 2′, 2′-difluorodeoxycytidine (gemcitabine)-resistant human ovarian cancer cell line AG6000 to standard and investigational drugs. Eur J Cancer 36:1974–1983. doi:10.1016/S0959-8049(00)00246-X
Schirmer M, Stegmann AP, Geisen F, Konwalinka G (1998) Lack of cross-resistance with gemcitabine and cytarabine in cladribine-resistant HL60 cells with elevated 5′-nucleotidase activity. Exp Hematol 26:1223–1228
Acknowledgement
This Paper was supported by the Dong-A University Research Fund.
Gemcitabine (Gemzar®) was kindly provided by Lilly Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S.Y., Kim, W.S., Lee, D.H. et al. Phase II study of Gemcitabine for treatment of patients with advanced stage marginal zone B-cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) trial. Invest New Drugs 28, 171–177 (2010). https://doi.org/10.1007/s10637-009-9260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9260-6