Summary
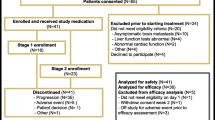
Purpose: Enzastaurin is a potent, serine-threonine kinase inhibitor which selectively targets PKCβ and PI3K/AKT signaling pathways to reduce cell proliferation, induce apoptosis, and inhibit angiogenesis. As PKCβ and PI3K/AKT signaling are both involved in breast cancer pathogenesis, this phase II study evaluated the efficacy and toxicity of enzastaurin in previously treated patients with metastatic breast cancer (MBC). Patients and Methods: Eligible patients had histologically confirmed MBC with measurable disease, and must have received prior anthracycline and taxane chemotherapy, but not more than two prior regimens for MBC. Human epidermal growth factor 2 (HER2)-positive patients must have progressed on prior trastuzumab therapy. Enzastaurin, 1,125-mg loading dose on day 1 followed by 500 mg daily, was administered orally in 28-day cycles. Response was assessed every 2 cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Results: Twenty-one patients enrolled between November 2006 and September 2007. Fourteen (66.7%) patients completed at least two cycles of therapy. No patients developed Grade 3/4 hematologic toxicity. Grade 3 nonhematologic toxicity was rare (<5%) and most commonly attributed to MBC progression. There were no objective responses and no patients with stable disease for ≥6 months. Median progression-free survival was 1.68 months (95%CI: 1.02, 1.74). Conclusions: Enzastaurin monotherapy was well tolerated, but demonstrated no activity in patients with heavily pretreated MBC.
Similar content being viewed by others
References
Goekjian PG, Jirousek MR (2001) Protein kinase C inhibitors as novel anticancer drugs. Expert Opin Investig Drugs 10:2117–2140 doi:10.1517/13543784.10.12.2117
O’Brian CA, Vogel VG, Singletary E et al (1989) Elevated protein kinase C expression in human breast tumor biopsies relative to normal breast tissue. Cancer Res 49:3215–3217
Gordge PC, Hulme MJ, Clegg RA et al (1996) Elevation of protein kinase A and protein kinase C activities in malignant as compared with normal human breast tissue. Eur J Cancer 32:2120–2126 doi:10.1016/S0959-8049(96)00255-9
Li H, Weinstein IB (2006) Protein kinase C β enhances growth and expression of cyclin D1 in human breast cancer cells. Cancer Res 66:11399–11408 doi:10.1158/0008-5472.CAN-06-2386
Gliki G, Wheeler-Jones C, Zachary I (2002) Vascular endothelial growth factor induces protein kinase C (PKC)-dependent Akt/PKB activation and phosphatidylinositol 3′-kinase-mediated PKC δ phosphorylation: role of PKC in angiogenesis. Cell Biol Int 26:751–759 doi:10.1016/S1065-6995(02)90926-1
Faul MM, Gillig JR, Jirousek MR et al (2003) Acyclic N-(azacycloalkyl)bisindolylmaleimides: isozyme selective inhibitors of PKCβ. Bioorg Med Chem Lett 13:1857–1859 doi::10.1016/S0960-894X(03)00286-5
Ma S, Rosen ST (2007) Enzastaurin. Curr Opin Oncol 19:590–595
Hanauske AR, Oberschmidt O, Hanauske-Abel H et al (2007) Antitumor activity of enzastaurin (LY317615.HCl) against human cancer cell lines and freshly explanted tumors investigated in in vitro soft-agar cloning experiments. Invest New Drugs 25:205–210 doi:10.1007/s10637-007-9038-7
Teicher BA, Alvarez E, Menon K et al (2002) Antiangiogenic effects of a protein kinase Cbeta-selective small molecule. Cancer Chemother Pharmacol 49:69–77 doi:10.1007/s00280-001-0386-2
Keyes KA, Mann L, Sherman M et al (2004) LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol 53:133–140 doi:10.1007/s00280-003-0713-x
Graff JR, McNulty AM, Hanna KR et al (2005) The protein kinase Cβ-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res 65:7462–7469 doi:10.1158/0008-5472.CAN-05-0071
Moreau A-S, Jia X, Ngo HT et al (2007) Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenström macroglobulinemia. Blood 109:4964–4972 doi:10.1182/blood-2006-10-054577
Carducci MA, Musib L, Kies MS et al (2006) Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol 24:4092–4099 doi:10.1200/JCO.2005.05.3447
Leong S, Camidge R, Eckhardt G et al (2006) A phase I dose-escalation and pharmacokinetic study of enzastaurin combined with capecitabine in patients with advanced cancer. ASCO 24S:2048
Robertson MJ, Kahl BS, Vose JM et al (2007) Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 25:1741–1746 doi:10.1200/JCO.2006.09.3146
Oh Y, Herbst R, Burris H et al (2008) Enzastaurin, an oral serine/threonine kinase inhibitor as second- or third-line therapy of non-small cell lung cancer (NSCLC). J Clin Oncol 26:7543–1141 doi:10.1200/JCO.2007.14.3685
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216 doi:10.1093/jnci/92.3.205
National Cancer Institute (2006) Common terminology criteria for adverse events, version 3.0. Available at http://ctep.cancer.gov/forms/CTCAEv3.pdf
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10 doi:10.1016/0197-2456(89)90015-9
Kaplan E, Meier P (1958) Nonparametric estimation of incomplete observations. J Am Stat Assoc 53:457–481 doi:10.2307/2281868
Rademaker-Lakhai J, Beerepoot L, Mehra N et al (2007) Phase I pharmacokinetic and pharmacodynamic study of the oral protein kinase C β-inhibitor enzastaurin in combination with gemcitabine and cisplatin in advanced cancer. Clin Cancer Res 13:4474–4481 doi:10.1158/1078-0432.CCR-06-2912
Cobleigh MA, Vogel CL, Tripathy D et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639–2648
Cobleigh MA, Langmuir VK, Sledge GW et al (2003) A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30(Suppl 16):117–124 doi:10.1053/j.seminoncol.2003.08.013
Acknowledgements
This trial was supported by Eli Lilly and Company, Indianapolis, IN. The authors thank Luna Musib and John Gill for their editorial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was sponsored by Eli Lilly and Company, Indianapolis, Indiana.
Rights and permissions
About this article
Cite this article
Mina, L., Krop, I., Zon, R.T. et al. A phase II study of oral enzastaurin in patients with metastatic breast cancer previously treated with an anthracycline and a taxane containing regimen. Invest New Drugs 27, 565–570 (2009). https://doi.org/10.1007/s10637-009-9220-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9220-1