Summary
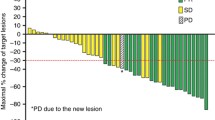
Introduction: Patients with metastatic colorectal cancer who progress on standard chemotherapy have limited treatment options. New and effective drugs are needed for these patients. Romidepsin is a histone deacetylase inhibitor that can alter chromatin structure and gene transcription leading to multiple changes in cellular protein production. This may result in cell cycle arrest and tumor growth inhibition. Romidepsin has shown anti-proliferative activity in vitro against multiple mouse and human tumor cell lines and in vivo in human tumor xenograft models. Patients and methods: Patients were required to have pathologically verified, measurable, metastatic or locally advanced colorectal cancer that was surgically unresectable. They must have failed either one or two prior chemotherapy regimens, had performance status of 0–1, adequate bone marrow, renal and hepatic function, and no significant cardiac disease. Patients were treated with romidepsin at a dose of 13 mg/m2 as a 4-h iv infusion on days 1, 8, and 15 of a 28-day cycle. The study had a two stage design. The primary objective of the study was to determine the confirmed response probability in this group of patients treated with romidepsin. Results: Twenty-eight patients were registered to the study, two of whom were ineligible. One eligible patient refused all treatment and was not analyzed. For the 25 remaining patients, performance status was 0 in 16 patients and 1 in nine patients. Ten patients had received one prior chemotherapy regimen and fifteen 2 prior regimens. Out of the 25 eligible and analyzable patients accrued in the first stage of the protocol, no objective responses were observed and the study was permanently closed. Four patients had stable disease as the best response. Twenty-five patients were assessed for toxicity. No grade 4 or greater toxicities were seen. Fourteen of the 25 patients experienced grade 3 toxicities the most common of which were fatigue or anorexia. Conclusion: Romidepsin at this dose and schedule is ineffective in the treatment of patients with metastatic colorectal cancer after prior chemotherapy. Future trials might evaluate combinations of romidepsin with chemotherapeutic or other agents.


Similar content being viewed by others
References
Ueda H, Nakajima H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M (1994) FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot 47:301–310
Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M (1994) Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968, on Ha-ras transformed NIH3T3 Cells. Biosci Biotechnol Biochem 58:1579–1583
Fecteau K, Mei J, Wang HC (2002) Differential modulation of signaling pathways and apoptosis of ras-transformed lOTl/2 cells by the depsipeptide FR901228. J Pharmacol Exp Ther 300:890–899 doi:10.1124/jpet.300.3.890
Sandor V, Senderowicz A, Mertins S, Sackett D, Sausville E, Blagoskionny MV, Bates SE (2000) P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer 83:817–825 doi:10.1054/bjoc.2000.1327
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202 doi:10.1038/35106079
Weidle UH, Grossman A (2000) Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res 20:1471–1485
Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S (1998) FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res 241:126–133 doi:10.1006/excr.1998.4027
Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB (1999) Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 21:103–107 doi:10.1038/5047
Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T (2001) Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab 86:3430–3435 doi:10.1210/jc.86.7.3430
Kitazono M, Goldsmith ME, Aikou T, Bates S, Fojo T (2001) Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res 61:6328–6330
Weiser TS, Ohnmacht GA, Guo ZS, Fischette MR, Chen GA, Hong JA, Nguyen DM, Schrump DS (2001) Induction of MAGE-3 expression in lung and esophageal cancer cells. Ann Thorac Surg 71:295–301 doi:10.1016/S0003-4975(00)02421-8
Weiser TS, Guo ZS, Ohnmacht GA, Parkhurst ML, Tong-On P, Marincola FM, Fischette MR, Yu X, Chen GA, Hong JA, Stewart JH, Nguyen DM, Rosenberg SA, Schrump DS (2001) Sequential 5-Aza-2′-deoxycytidine-Depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother 24:151–161 doi:10.1097/00002371-200103000-00010
Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, Villalona-Calero MA, Plass C, Otterson GA (2001) Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene 20:7787–7796 doi:10.1038/sj.onc.1204970
Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, Sun J, Yu Y, Zhou W, Zheng Q, Wu M, Otterson GA, Zhu WG (2006) Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1). Mol Cell Biol 26:2782–2790 doi:10.1128/MCB.26.7.2782-2790.2006
Zhu WG, Otterson GA (2003) The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents 3:187–199 doi:10.2174/1568011033482440
Kwon HJ, Kim MS, Kim MJ, Nakajima H, Kim KW (2002) Histone deacetylase inhibitor FK228 inhibits tumor angiogenesis. Int J Cancer 97:290–296 doi:10.1002/ijc.1602
Murata M, Towatari M, Kosugi H, Tanimoto M, Ueda R, Saito H, Naoe T (2000) Apoptotic cytotoxic effects of a histone deacetylase inhibitor, FK228, on malignant lymphoid cells. Jpn J Cancer Res 91:1154–1160
Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS (2002) Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst 94:504–513
Rajgolikar G, Chan KK, Wang HC (1998) Effects of a novel antitumor depsipeptide, FR901228, on human breast cancer cells. Breast Cancer Res Treat 51:29–38 doi:10.1023/A:1006091014092
Ueda H, Manda T, Matsumoto S, Mukumoto S, Nishigaki F, Kawamura I, Shimomura K (1994) FR9O1228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. III. Antitumor activities on experimental tumors in mice. J Antibiot 47:315–323
Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, Brooks R, Piekarz RL, Tucker E, Figg WD, Chan KK, Goldspiel B, Fojo AT, Balcerzak SP, Bates SE (2002) Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res 8:718–728
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate response to treatment in solid tumors. J Natl Cancer Inst 92:205–216 doi:10.1093/jnci/92.3.205
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51 doi:10.1038/nrc1779
Marchion D, Munster P (2007) Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther 7:583–598 doi:10.1586/14737140.7.4.583
Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, Sachdev V, Fojo T, Bates SE (2006) Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res 12:3762–3773 doi:10.1158/1078-0432.CCR-05-2095
Glaser KB (2007) HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol 74:659–671 doi:10.1016/j.bcp.2007.04.007
Acknowledgements
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA58416, CA74647, CA58861, CA105409, CA45807, CA35178, CA45560, CA46441, CA12644, CA20319, CA45808, CA35128, CA58723, CA22433, CA46113, CA105409, CA46113 CA20319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitehead, R.P., Rankin, C., Hoff, P.M.G. et al. Phase II trial of romidepsin (NSC-630176) in previously treated colorectal cancer patients with advanced disease: a Southwest Oncology Group study (S0336). Invest New Drugs 27, 469–475 (2009). https://doi.org/10.1007/s10637-008-9190-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9190-8