Summary
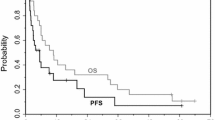
Purpose: This phase I trial assessed the safety and the maximum tolerated dose of capecitabine given for 10 days prior to a combination of cisplatin and irinotecan in patients with advanced solid malignancies. It also evaluated the changes in cisplatin DNA adducts induced by capecitabine. Patients and Methods: Patients with refractory solid tumors who had not failed 5-fluorouracil (5-FU) analogs or topoisomerase I inhibitors were eligible. All cohorts of patients first received a 28-day cycle of cisplatin and irinotecan. Both drugs were given at a dose of 50 mg/m2 intravenously on day 1, followed by irinotecan on days 8 and 15 at the same dose. The first cycle served as an internal control. Starting from the second cycle, patients received increasing doses per cohort of capecitabine from day 1 to 10 of each cycle, followed by cisplatin on day 11 and irinotecan on days 11, 18 and 25, both at same doses as the first cycle. Cycles were repeated every 38 days. The starting dose of capecitabine was 500 mg/m2/day which was escalated by 250 mg/m2/day in the subsequent cohort of patients to reach the maximum tolerated dose (MTD). Later, additional patients were treated at the MTD of capecitabine to further evaluate the safety, pharmacodynamics, and tumor response. Patients blood was tested for cisplatin-DNA adducts to determine the impact of capecitabine on cisplatin-based therapy. Results: Fifteen patients received at least 2 cycles of treatment. At 1,250 mg/m2, two DLT of prolonged neutropenia of grade ≥3 were observed. The MTD for capecitabine was thus determined to be 1000 mg/m2/day. Fatigue and diarrhea of grade 1 or 2 were the most frequent toxicities at this dose level. No significant hematologic toxicity was observed at the MTD. Two complete and three partial remissions were observed. Four of the responders had received a platinum agent and/or 5-FU in the past. Conclusions: A sequential treatment with capecitabine followed by cisplatin and irinotecan is well tolerated and demonstrates clinical activity in patients with advanced solid malignancies. The influence of capecitabine, if any, on the efficacy of the cisplatin-irinotecan combination is not related to a variation in cisplatin-DNA adducts.



Similar content being viewed by others
References
Noda K, Nishiwaki Y, Kawahara M et al (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91 doi:10.1056/NEJMoa003034
Boku N, Ohtsu A, Shimada Y et al (1999) Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 17:319–323
Sugiyama T, Yakushiji M, Kamura T et al (2002) Irinotecan (CPT-11) and cisplatin as first-line chemotherapy for advanced ovarian cancer. Oncology 63:16–22 doi:10.1159/000065715
Chitapanarux I, Tonusin A, Sukthomya V et al (2003) Phase II clinical study of irinotecan and cisplatin as first-line chemotherapy in metastatic or recurrent cervical cancer. Gynecol Oncol 89:402–407 doi:10.1016/S0090-8258(03)00174-4
Saltz LB, Cox JV, Blanke C et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914 see commentsdoi:10.1056/NEJM200009283431302
Baldwin J (2002) FDA evaluating oxaliplatin for advanced colorectal cancer treatment. J Natl Cancer Inst 94:1191–1193
Misset JL, Bleiberg H, Sutherland W et al (2000) Oxaliplatin clinical activity: a review. Crit Rev Oncol Hematol 35:75–93 doi:10.1016/S1040-8428(00)00070-6
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Souglakos J, Mavroudis D, Kakolyris S et al (2002) Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol 20:2651–2657 doi:10.1200/JCO.2002.08.015
Hughes LL, Luengas J, Rich TA et al (1992) Radiosensitization of cultured human colon adenocarcinoma cells by 5-fluorouracil: effects on cell survival, DNA repair, and cell recovery. Int J Radiat Oncol Biol Phys 23:983–991
Alderden RA, Hall MD, Hambley TW (2006) The discovery and development of cisplatin. JCE 83:728–734
Mathijssen RH, Loos WJ, Verweij J et al (2002) Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Curr Cancer Drug Targets 2:103–123 doi:10.2174/1568009023333890
Jacob S, Aguado M, Fallik D et al (2001) The role of the DNA mismatch repair system in the cytotoxicity of the topoisomerase inhibitors camptothecin and etoposide to human colorectal cancer cells. Cancer Res 61:6555–6562
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors.European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 doi:10.1093/jnci/92.3.205
Author information
Authors and Affiliations
Corresponding author
Additional information
Previous presentations:
C. F. Verschraegen, F. C. Lee, I. Rabinowitz, A. Mangalik, C. Jennings, A. Maestas, Z. Shen. Phase I and translational study of capecitabine, cisplatin, and irinotecan in patients with solid tumors. Proc ASCO, A2114, 2004
H. Sayar, C. Verschraegen, H. Smith, I. Rabinowitz, F. C. Lee, Z. Shen. Phase I study of capecitabine in combination with cisplatin and irinotecan in patients with advanced solid malignancies. Proc ASCO, AB-31962, 2008
Support:
Supported by the Oxnard Foundation; Period of Support: 07/01/2003–06/30/2005
Rights and permissions
About this article
Cite this article
Sayar, H., Shen, Z., Lee, S.J. et al. Phase I study of capecitabine in combination with cisplatin and irinotecan in patients with advanced solid malignancies. Invest New Drugs 27, 153–158 (2009). https://doi.org/10.1007/s10637-008-9172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9172-x