Summary
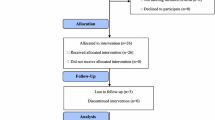
The purpose of this study was to evaluate the efficacy (progression free survival (PFS) and response rate) and safety of vinorelbine and trastuzumab combination chemotherapy in patients with HER2-overexpressing, metastatic breast cancer as a first line chemotherapy regimen. Patients with histologically confirmed, HER2-positive (immunohistochemistry (ICH) 3+, or 2+ and FISH+) metastatic breast cancer who had nor received prior vinorelbine or anti-HER2 therapy in the adjuvant setting, received at least eight weeks of vinorelbine i.v. (25 mg/g weekly) and trastuzumab (4 mg/kg on day 1 followed by 2 mg/kg weekly). Forty-one women from six participating centers were enrolled into the trial. The overall response rate, was 43.9% (18 of 41 patients), (CI 28–60.3%), 30% of patients were progression free after 1 year. Four patients reached complete remission, 14 partial remission and five had stable disease for at least 18 weeks. Six patients developed primary progression. 35 patients (85%) experienced progression after a median time of 235 days. Therapy was in general well-tolerated. There were two CTC grade 4 infusion syndromes and two patients experienced cardiotoxicity at least grade 2. This phase II trial of vinorelbine and trastuzumab demonstrated an effective and well-tolerated regimen with a favourable safety profile.


Similar content being viewed by others
References
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10. doi:10.1016/0197-2456(89)90015-9
Berger MS et al (1988) Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res 48(5):1238–1243
Slamon DJ et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182. doi:10.1126/science.3798106
Slamon DJ et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712. doi:10.1126/science.2470152
Vogel CL et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726. doi:10.1200/JCO.20.3.719
Cobleigh MA et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Baselga J et al (1999) Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol 26(4 Suppl 12):78–83
Slamon DJ et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. doi:10.1056/NEJM200103153441101
Osoba D et al (1999) Health-related quality of life in women with metastatic breast cancer treated with trastuzumab (Herceptin). Semin Oncol 26(4 Suppl 12):84–88
Pegram MD et al (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18(13):2241–2251. doi:10.1038/sj.onc.1202526
Pegram MD et al (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96(10):739–749
Pietras RJ et al (1998) Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 17:2235–2249. doi:10.1038/sj.onc.1202132
Gianni L et al (1995) Paclitaxel in metastatic breast cancer: a trial of two doses by a 3-hour infusion in patients with disease recurrence after prior therapy with anthracyclines. J Natl Cancer Inst 87(15):1169–1175. doi:10.1093/jnci/87.15.1169
Seidman AD et al (1995) Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol 13(10):2575–2581
Suzuki Y et al (2003) Combination of trastuzumab and vinorelbine in metastatic breast cancer. Jpn J Clin Oncol 33(10):514–517. doi:10.1093/jjco/hyg101
Burstein HJ et al (2003) Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumour markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol 21(15):2889–2895. doi:10.1200/JCO.2003.02.018
Winer EP, Burstein HJ (2001) New combinations with Herceptin in metastatic breast cancer. Oncology 61(Suppl 2):50–57. doi:10.1159/000055402
Burstein HJ et al (2007) Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbin or taxane study. Cancer 110(5):965–972. doi:10.1002/cncr.22885
Franquesa R, Centelles M, Villadiego K (2005) A multicenter study of trastuzumab (H) and vinorelbine (N) as first and second line therapy for patients with HER2-positive metastatic breast cancer (HER2+ MBC). ASCO Meeting Abstracts, 868
Jahanzeb M et al (2002) Phase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancer. Oncologist 7(5):410–417
Burstein HJ et al (2001) Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 19(10):2722–2730
Porta V, Martin M, Gil M (2004) Evaluation of vinorelbine (N) and trastuzumab (H) as first line therapy for HER2-positive metastatic breast cancer (HER2+ MBC): impact on clinical response and cardiac function. ASCO Meeting Abstracts: 636
Bartsch R, Wenzel C, Altorjai G (2007) Results from an observational trial with oral vinorelbine and trastuzumab in advanced breast cancer. Breast Cancer Res Treat 102(3):375–381. doi:10.1007/s10549-006-9342-5
Catania C, Medici M, Magni E (2007) Optimizing clinical care of patients with metastatic breast cancer: a new oral vinorelbine plus trastuzumab combination. Ann Oncol 18(12):1969–1975. doi:10.1093/annonc/mdm372
Acknowledgments
We thank all participating patients and attending medical staff in the different centers, Klaus Pahnke, Büro für Statistik, Reinheim, Germany for statistical analyses and the monitors of GSO-Gesellschaft für Studienmanagement und Onkologie mbH, Hamburg, Germany. Financial support was provided by Pierre Fabre Pharma GmbH, Freiburg, Germany and Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schilling, G., Bruweleit, M., Harbeck, N. et al. Phase II trial of vinorelbine and trastuzumab in patients with HER2-positive metastatic breast cancer. A prospective, open label, non-controlled, multicenter phase II trial (to investigate efficacy and safety of this combination chemotherapy). Invest New Drugs 27, 166–172 (2009). https://doi.org/10.1007/s10637-008-9166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9166-8