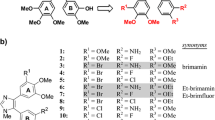
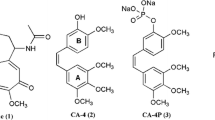
Summary
Colchicine site tubulin inhibitors are currently developed as vascular disrupting agents (VDAs). However, they were found to have cardiotoxicity in clinical trials. To overcome the problem, we developed a stilbene derivative, cis-3, 4′, 5-trimethoxy-3′-aminostilbene (stilbene 5c), which is highly potent and has no bone marrow and cardiac toxicity in mice. Here we attempt to optimize stilbene 5c using computer-based drug design and synthesize derivatives with benzimidazole or indole group. Biological evaluation showed that they are weaker than stilbene 5c without better water solubility. Alternative approach was thus adopted to make prodrugs of stilbene 5c. A water-soluble prodrug PD7 was synthesized by addition of a morpholino group with carbamate linkage to the amino group of stilbene 5c. In vitro studies show that PD7 induces mitotic arrest and disrupts microtubule similar to stilbene 5c. The cell signaling events in Cdc2, p53, Akt, and aurora kinase are similar in cells treated with stilbene 5c, CA4 or PD7, suggesting that they share the same mechanism. Although PD7 is less effective than stilbene 5c in vitro, the biological activity of PD7 as a single agent is similar to that of stilbene 5c. Combination of PD7 with VEGF inhibitor bevacizumab significantly enhances the therapeutic efficacy of PD7 in mouse xenograft model. These data suggest that PD7 could be a good candidate for further pre-clinical and clinical development as a new VDA for cancer therapy.





Similar content being viewed by others
References
Neri D, Bicknell R (2005) Tumour vascular targeting. Nat Rev Cancer 5(6):436–446
Folkman J, Browder T, Palmblad J (2001) Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost 86(1):23–33
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6(4):273–286
Collins TS, Hurwitz HI (2005) Targeting vascular endothelial growth factor and angiogenesis for the treatment of colorectal cancer. Semin Oncol 32(1):61–68
Shih T, Lindley C (2006) Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 28(11):1779–1802
Garcia JA, Rini BI (2007) Recent progress in the management of advanced renal cell carcinoma. CA Cancer J Clin 57(2):112–125
Rini BI (2007) Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res 13(4):1098–1106
O’Hanlon LH (2005) Taking down tumors: vascular disrupting agents entering clinical trials. J Natl Cancer Inst 97(17):1244–1245
Siemann DW, Chaplin DJ, Horsman MR (2004) Vascular-targeting therapies for treatment of malignant disease. Cancer 100(12):2491–2499
Thorpe PE (2004) Vascular targeting agents as cancer therapeutics. Clin Cancer Res 10(2):415–427
Dowlati A et al (2002) A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 62(12):3408–3416
Stevenson JP et al (2003) Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 21(23):4428–4438
Rustin GJ et al (2003) Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol 21(15):2815–2822
Anderson HL et al (2003) Assessment of pharmacodynamic vascular response in a phase I trial of combretastatin A4 phosphate. J Clin Oncol 21(15):2823–2830
Beerepoot LV et al (2006) Phase I clinical evaluation of weekly administration of the novel vascular-targeting agent, ZD6126, in patients with solid tumors. J Clin Oncol 24(10):1491–1498
Roberti M et al (2003) Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem 46(16):3546–3554
Cao TM et al (2008) Stilbene derivatives that are colchicine site microtubule inhibitors have anti-leukemic activity and minimal systemic toxicity. Am J Hematol 83(5):390–397
Durrant D et al (2008) cis-3, 4′, 5-trimethoxy-3′-aminostilbene disrupts tumor vascular perfusion without damaging normal organ perfusion. Cancer Chemother Pharmacol DOI 10.1007/s00280-008-0726-6
Tozer GM et al (1999) Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res 59(7):1626–1634
Malcontenti-Wilson C et al (2001) Combretastatin A4 prodrug study of effect on the growth and the microvasculature of colorectal liver metastases in a murine model. Clin Cancer Res 7(4):1052–1060
Leu YL, Roffler SR, Chern JW (1999) Design and synthesis of water-soluble glucuronide derivatives of camptothecin for cancer prodrug monotherapy and antibody-directed enzyme prodrug therapy (ADEPT). J Med Chem 42(18):3623–3628
Lougerstay-Madec R et al (1998) Synthesis of self-immolative glucuronide-based prodrugs of a phenol mustard. Anticancer Drug Des 13(8):995–1007
Tozer GM, Kanthou C, Baguley BC (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5(6):423–435
Thomson P et al (2006) Synthesis and biological properties of bioreductively targeted nitrothienyl prodrugs of combretastatin A-4. Mol Cancer Ther 5(11):2886–2894
Bakker M et al (1998) Broad phase II and pharmacokinetic study of methoxy-morpholino doxorubicin (FCE 23762-MMRDX) in non-small-cell lung cancer, renal cancer and other solid tumour patients. Br J Cancer 77(1):139–146
Kuratsu JI et al (2000) Phase II trial of pre-irradiation KRN8602 (MX2) in malignant glioma patients. J Neurooncol 48(2):145–149
Takemoto Y et al (1999) A prospective randomized trial of KRN8602 and cytosine arabinoside vs. daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. The KRN8602 Leukemia Study Group. Int J Hematol 70(1):20–25
Kuratsu J et al (1999) A phase II study of KRN8602(MX2), a novel morpholino anthracycline derivative, in patients with recurrent malignant glioma. J Neurooncol 42(2):177–181
Lau DH, Duran GE, Sikic BI (1992) Characterization of covalent DNA binding of morpholino and cyanomorpholino derivatives of doxorubicin. J Natl Cancer Inst 84(20):1587–1592
Zhang S, Hemmerich P, Grosse F (2007) Centrosomal localization of DNA damage checkpoint proteins. J Cell Biochem 101(2):451–465
Castedo M et al (2004) The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene 23(25):4353–4361
Durrant D et al (2008) Mechanism of cell death induced by cis-3, 4′, 5-trimethoxy-3′-aminostilbene in ovarian cancer. Gynecol Oncol PMID: 18433847
Yoshida K, Liu H, Miki Y (2006) Protein kinase C delta regulates Ser46 phosphorylation of p53 tumor suppressor in the apoptotic response to DNA damage. J Biol Chem 281(9):5734–5740
Komiyama S et al (2004) Potentiality of DNA-dependent protein kinase to phosphorylate Ser46 of human p53. Biochem Biophys Res Commun 323(3):816–822
Cecchinelli B et al (2006) Ser58 of mouse p53 is the homologue of human Ser46 and is phosphorylated by HIPK2 in apoptosis. Cell Death Differ 13(11):1994–1997
Rinaldo C et al (2007) MDM2-regulated degradation of HIPK2 prevents p53Ser46 phosphorylation and DNA damage-induced apoptosis. Mol Cell 25(5):739–750
Oricchio E et al (2006) ATM is activated by default in mitosis, localizes at centrosomes and monitors mitotic spindle integrity. Cell Cycle 5(1):88–92
Ditchfield C et al (2003) Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol 161(2):267–280
Morrow CJ et al (2005) Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J Cell Sci 118(Pt 16):3639–3652
Shaked Y et al (2006) Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 313(5794):1785–1787
Acknowledgement
The work was supported by Massey Cancer Center and Virginia Commonwealth University pilot program (R.M.L.). The flow cytometry and confocal microscope are supported by Cancer Center Core Grant (P30 CA16059). We thank Drs. Ming Zhao and Michelle Rudek (the Sidney Kimmel Comprehensive Cancer Center, the Johns Hopkins University) for LC/MS/MS study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durrant, D.E., Richards, J., Tripathi, A. et al. Development of water soluble derivatives of cis-3, 4′, 5-trimethoxy-3′-aminostilbene for optimization and use in cancer therapy. Invest New Drugs 27, 41–52 (2009). https://doi.org/10.1007/s10637-008-9139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9139-y