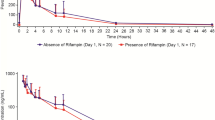
Summary
A phase I study was performed to determine the safety and pharmacokinetics of XK469R in patients with refractory acute leukemia. The study aimed to determine the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of XK469R given intravenously over 30 to 60 min on days 1, 3, and 5 of a 21 day cycle. Patients were treated in successive cohorts of six until DLT was observed. Once the MTD was determined, an additional cohort of six patients was enrolled at the previous dose level and that dose was considered the recommended phase 2 dose (RPTD). Forty-six patients were treated at dose levels of 1,400, 1,750, 2,200, and 2,750 mg. The DLTs were: mucositis, colitis and hyperbilirubinemia. Reversible myelosuppression was noted at all dose levels. One (2%) of 42 patients achieved a complete remission and five patients (11%) had hematologic improvement. The half-life of the drug was long with a mean value of 48 h. The mean clearance was 206 mL/h with a coefficient of variation of 32%. No correlation was observed between the development of DLT and pharmacokinetics. The RTPD is 1,750 mg. XK469R induced hematological responses in patients with refractory leukemia at tolerable doses.


Similar content being viewed by others
References
Aribi A, Ravandi F, Giles F (2006) Novel agents in acute myeloid leukemia. Cancer J 12:77–91
LoRusso PM, Parchment R, Demchik L et al (1998) Preclinical antitumor activity of XK469 (NSC 656889). Invest New Drugs 16:287–296
Gao H, Huang KC, Yamasaki EF et al (1999) XK469, a selective topoisomerase IIbeta poison. Proc Natl Acad Sci U S A 96:12168–12173
Kessel D, Horwitz JP (2001) Pro-apoptotic interactions between XK469 and the peripheral benzodiazepine receptor. Cancer Lett 168:141–144
Lin H, Subramanian B, Nakeff A et al (2002) XK469, a novel antitumor agent, inhibits signaling by the MEK/MAPK signaling pathway. Cancer Chemother Pharmacol 49:281–286
Ding Z, Parchment RE, LoRusso PM et al (2001) The investigational new drug XK469 induces G(2)-M cell cycle arrest by p53-dependent and -independent pathways. Clin Cancer Res 7:3336–3342
Ding Z, Zhou JY, Wei WZ et al (2002) Induction of apoptosis by the new anticancer drug XK469 in human ovarian cancer cell lines. Oncogene 21:4530–4538
Polin L, White K, Kushner J et al (2002) Preclinical efficacy evaluations of XK-469: dose schedule, route and cross-resistance behavior in tumor bearing mice. Invest New Drugs 20:13–22
Sprague E, Undevia SD, Innocenti F et al (2004) A dose escalation study of the quinolzaline antitumor agent R(+)XK469 (XK) in patients with refractory solid tumors. Proc Am Soc Clin Oncol 22:2021
Zheng H, Covey JM, Tosca PJ et al (2002) Chiral high-performance liquid chromatographic analysis of the enantiomers of XK469, a new antitumor agent, in plasma and urine. J Pharm Biomed Anal 28:287–294
Acknowledgements
The authors wish to thank Dawn Spearmon for her administrative assistance in the preparation of this manuscript. We would also like to acknowledge the hard work of Margaret Green, RN, whose research nursing expertise was invaluable.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by CA069852 and CA062461 and by the University of Chicago Cancer Research Center, P30 CA014599-32 S2.
Rights and permissions
About this article
Cite this article
Stock, W., Undevia, S.D., Bivins, C. et al. A phase I and pharmacokinetic study of XK469R (NSC 698215), a quinoxaline phenoxypropionic acid derivative, in patients with refractory acute leukemia. Invest New Drugs 26, 331–338 (2008). https://doi.org/10.1007/s10637-008-9129-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9129-0