Summary
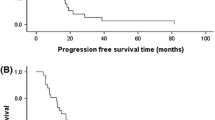
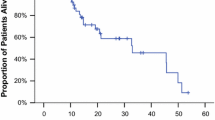
Gemcitabine and cisplatin are the active agents in metastatic breast cancer pretreated with anthracycline and/or taxane as a second line treatment. The present study was designed to assess the efficacy and safety of this regimen given biweekly schedule in these patients. Twenty-seven women, median age 57, with metastatic breast cancer previously treated with anthracycline and taxane were eligible for enrollment. Gemcitabine was administered intravenously on days 1 and 15 at a dose of 2,000 mg/m2 and Cisplatin was given intravenously on day 1 and 15 at a dose of 50 mg/m2. Treatment cycles were repeated on an outpatient basis every 28 days. Of all 27 evaluable patients, the overall response rate was 26% (7 of 27; 95% CI: 11–46%) with seven all partial responses. The stable diseases were found in 9 (33%) patients. At the time of last follow-up, 11 (41%) of the patients died of their disease progression. The median overall survival duration was 7.4 ± 2.8 months. The 1-year overall survival rate was 46.9% ± 12.3. Hematological toxicity was not found as the principal dose-limiting toxicity. Severe (grade III/IV) neutropenia was observed only one (4%) patients. No patient was complicated by febrile neutropenia and G-CSF usage was not performed. Grade III and IV anemia were seen in only 4 (15%) and thrombocytopenia was noted only one (4%) patients. Severe hepatic (n = 2) and renal toxicity (n = 1) were observed and these all recovered completely without complication. Several other severe non-hematological side effects were managed easily. Permanent dose reductions were necessary in 9 (33%) patients and chemotherapy administration was also delayed in 7 (26%) patients because of delayed both hematological and non-hematological toxicity recovery. Treatment was discontinued in one (4%) patient due to severe fatigue and deteriorating performance status. In conclusion, gemcitabine and cisplatin combination therapy with this biweekly schedule and dosage is moderately active and extremely safe in patients with metastatic breast cancer previously treated with anthracycline and taxanes.

Similar content being viewed by others
References
Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, Harris AL (1995) Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol 13:2731–2736
Schmid P, Akrivakis K, Flath B, Grosse Y, Sezer O, Mergenthaler HG, Possinger K (1999) Phase II trial of gemcitabine as prolonged infusion in metastatic cancer. Anticancer Drugs 10:625–631
Brodowicz T, Kostler WJ, Moslinger R, Tomek S, Vaclavik I, Hersovici V, Witschke C, Steger GG, Wein W, Seifert M, Kubista E, Zielinski CC (2000) Single-agent gemcitabine as second- and third-line treatment in metastatic breast cancer. Breast 9:338–342
Gerson R, Serrano OA, Villalobos A, Ortiz C, Sanchez-Forgach R (2000) Gemcitabine response in advanced breast cancer in relation to immunohistochemical factors (Abstract). Proc Am Soc Clin Oncol 19: (abstract 572)
Spielmann M, Llombart-Cussac A, Kalla S, Espie M, Namer M, Ferrero JM, Dieras V, Fumoleau P, Cuvier C, Perrocheau G, Ponzio A, Kayitalire L, Pouillart P (2001) Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology 60:303–307
Valerio M, Cicero G, Armata M, Bajardi E, Crosta A, Badalamenti G, Arcara C, Agosta G, Vieni S, Latteri F, Russo A, Gulotta G, Gebbia N (2001) Gemcitabine (G) in pretreated breast cancer (BC) (Abstract). Proc Am Soc Clin Oncol 20: (abstract 1953)
Burch PA, Mailliard J, Hillman D, Perez E, Krook J, Rowland K (2000) Phase II study of gemcitabine and cisplatin in patients with metastatic breast cancer (MBC) and failure on prior chemotherapy: a North Central Treatment Group trial (Abstract). Breast Cancer Res Treat 64:81 (abstract 322)
Chaudhry S, Abdel-Rahman H, Patil R, Mansour R, Mills G, Burton GV (2000) Prospective phase II study of weekly cisplatin-gemcitabine in refractory metastatic breast cancer (RM-BC) (Abstract). Proc Am Soc Clin Oncol 19: (abstract 430)
Galvez CA, Calmarini F, Curie M (2000) Monthly cisplatin (C) and gemcitabine (G) as second line chemotherapy for patients with advanced breast cancer (Abstract). Breast Cancer Res Treat 64(1):81
Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans SS (2000) Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol 18:2245–2249
Doroshow J, Tetef M, Margolin K, Somlo G, Frankel P, Longmate J, Synold T, Gandara D, Lrnz HJ, Albain K (2000) Significant activity of Gemcitabine (Gem) and Cisplatin (Ddp)in Both Heavily (H) and Minimally (M)-Pretreated Metastatic Breast Cancer (Mbc) Patients (Pts): A California Cancer Consortium/Loyola Univ. Chicago Trial (Abstract). Proc Am Soc Clin Oncol 19: (abstract 609H)
de la Pena HF, Tellez E, Bastarrachea J, Capdeville D (2001) Gemcitabine plus cisplatin as a palliative treatment in metastatic or refractory breasr cancer (Abstract). Proc Am Soc Clin Oncol 20: (abstract 1990)
Burch PA, Mailliard JA, Hillman DW, Perez EA, Krook JE, Rowland KM, Veeder MH, Cannon MW, Ingle JN (2005) Phase II study of gemcitabine plus cisplatin in patients with metastatic breast cancer: a North Central Cancer Treatment Group Trial. Am J Clin Oncol 28:195–200
Heinemann V, Stemmler HJ, Wohlrab A, Bosse D, Losem C, Kahlert S, Rautke G (2006) High efficacy of gemcitabine and cisplatin in patients with predominantly anthracycline- and taxane- pretreated metastatic breast cancer. Cancer Chemother Pharmacol 57:640–646
Seo JH, Oh SC, Choi CW, Kim BS, Shin SW, Kim YH, Kim JS, Kim AR, Lee JB, Koo BH (2007) Phase II study of a gemcitabine and cisplatin combination regimen in taxane resistant metastatic breast cancer. Cancer Chemother Pharmacol 59:269–274
Forastiere AA, Hakes TB, Wittes JT, Wittes RE (1982) Cisplatin in the treatment of metastatic breast carcinoma: a prospective randomized trial of two dosages schedules. Am J Clin Oncol 5:243–247
Martino S, Samal BA, Singhakowinta A, Yoshida S, Mackenzie M, Jain J, Vaitkevicius VK (1984) A phase II study of cis-diamminedichloroplatinum II for advanced breast cancer. Two dose schedules. J Cancer Res Clin Oncol 108:354–356
Decatris MP, Sundar S, O’Byrne KJ (2004) Platinum-based chemotherapy in metastatic breast cancer. Cancer Treat Rev 30:53–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tas, F., Guney, N., Derin, D. et al. Biweekly administration of gemcitabine and cisplatin chemotherapy in patients with anthracycline and taxane-pretreated metastatic breast cancer. Invest New Drugs 26, 363–368 (2008). https://doi.org/10.1007/s10637-007-9110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9110-3