Summary
Background
Selecting patients for phase I trials in order to investigate cytotoxic agents is challenging, since there is no clear and reliable guidance to estimate life expectancy among these patients. We retrospectively assessed prognostic factors in cancer patients screened for Phase1 trials between October 1997 and October 2002.
Methods
148 consecutive patients, screened for inclusion in phase I trials investigating cytotoxic agents, were included in the present study. 70 out of them actually received phase I trial regimens. Univariate and multivariate analysis were undertaken to determine the prognostic factors for overall survival (OS) from the date of screening.
Results
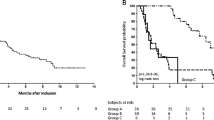
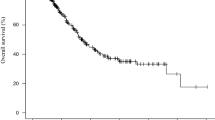
The median OS of the 148 patients was 5.7 months. Ninety-two percent of them had PS ≤ 1. The Cox model identified serum albumin <38 g/l [HR 2.51 (1.51–4.18), p = 0.0001] and lymphocyte count <700/mm3 [HR 2.27 (1.13–4.62), p = 0.024] as independent prognostic for overall survival. All patients presenting both prognostic factors died within 90 days.
Conclusions
We propose a simple model, easily obtained at the bedside, which can discriminate patients who are likely to be alive at 3 months and thus could be included in a phase 1 anti-cancer trial. This model now needs to be validated on an independent cohort.

Similar content being viewed by others
References
Weinfurt KP, Depuy V, Castel LD, Sulmasy DP, Schulman KA, Meropol NJ (2005) Understanding of an aggregate probability statement by patients who are offered participation in phase I clinical trials. Cancer 103:140–147
Kurzrock R, Benjamin RS (2005) Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med 352:930–932
Dillman RO, Koziol JA (1992) Phase I cancer trials: limitations and implications. Mol Biother 4:117–121
Lipsett MB (1995) On the nature and ethics of phase I trials. J Clin Oncol 13:1049–1051
Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R (1996) Therapeutic responses in phase I trials of antineoplastic agents. Cancer Treat Rep 70:1105–1115
Decoster G, Stein G, Holdener EE (1990) Response and toxic deaths in phase I clinical trials. Ann Oncol 1:175–181
Von Hoff DD, Turner J (1991) Response rates, duration of response and dose response effects in phase I studies. Invest New Drugs 9:115–121
Bachelot T, Ray-Coquard I, Catimel G et al (2000) Multivariate analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol 11:151–156
Kubo A, Corley DA (2006) body mass index and adenocarcinomas of the oesophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 15:872–878
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Estimation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170
Cox D (1972) Regression models and life tables (with discussion). J R Stat Soc B 34:187–220
Ray-Coquard I, Ghesquiere H, Bachelot T, Borg C, Biron P, Sebban C, et al (2001) Identification of patients at risk for early death after conventional chemotherapy in solid tumours and lymphomas. Br J Cancer 85:816–822
Claude L, Perol D, Ray-Coquard I, Petit T, Blay JY, Carrie C, et al (2005) Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol 76:334–339
Borg C, Ray-Coquard I, Philip I, Clapisson G, Bendriss-Vermare N, Menetrier-Caux C et al (2004) CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients cancer. Cancer 101:2675–2680
Seve P, Ray-Coquard I, Trillet-Lenoir V, Trillet-Lenoir V, Sawyer M, Hanson J, Broussole C et al (2006) Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer 107:2668–2670
Janisch L, Mick R, Schilsky RL, Vogelzang NJ, O’Brien S, Kut M et al (1994) Prognostic factors for survival in patients treated in phase I clinical trials. Cancer 74:1965–1973
Yamamoto N, Tamura T, Fukuoka M, Saijo N (1999) Survival and prognostic factors in lung cancer patients treated in phase I trials: Japanese experience. Int J Oncol 15:737–741
Daugherty CK, Ratain HJ, Grochowski E, Stocking C, Kodish E et al (1995) Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 13:1062
Coates RJ, Clark WS, Eley JW et al (1990) Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst 82:1684–1692
Liu SA, Tsai WC, Wong YK, Wong YK, Lin JC, Poon CK et al (2006) Nutritional factors and survival of patients with oral cancer. Head Neck 28:998–1007
Ayoub JP, Palmer JL, Huh Y, Cabanillas F, Younes A (1999) Therapeutic and prognostic implications of peripheral blood lymphopenia in patients with Hodgkin’s disease. Leuk Lymphoma 34:519–527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penel, N., Vanseymortier, M., Bonneterre, ME. et al. Prognostic factors among cancer patients with good performance status screened for phase I trials. Invest New Drugs 26, 53–58 (2008). https://doi.org/10.1007/s10637-007-9088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9088-x