Summary
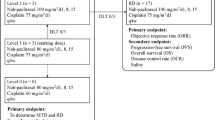
Aprinocarsen is a specific antisense oligonucleotide inhibitor of protein kinase C-α. This study aimed to evaluate the response rate to combination therapy with aprinocarsen, gemcitabine and cisplatin, in chemonaive patients with advanced/metastatic NSCLC. Secondary objectives included comparison of response rate, time to event efficacy parameters, and toxicities on the 2 treatment arms. Patients with stage IV, or stage IIIB disease (N3 and/or pleural/pericardial effusion), were randomized to either control or experimental arm. Patients on both arms received gemcitabine 1250 mg/m2 on days 1 and 8, and cisplatin 80 mg/m2 on day 1 of a 3-week cycle. Additionally, on the experimental arm, aprinocarsen was administered as 2 mg/kg continuous iv infusion on days 1–14, every 21 days. A total of 18 enrolled patients were randomized on the 2 arms. Further enrollment was terminated in March 2003 as a result of a phase III trial suggesting that aprinocarsen did not have an added survival benefit when combined with paclitaxel and carboplatin therapy in patients with NSCLC. Patients received a median of 4 cycles on control arm and 2.5 cycles on experimental arm. The response rate was 16.7% in the experimental arm and 44.4% in the control arm. Most frequent grade 3/4 toxicities were hematologic, with a higher incidence of thrombocytopenia in the experimental arm (87.5% vs. 33.3%). Despite the 14-day continuous infusion schedule, infection rate was not increased in the experimental arm. The present study did not show any advantage, in response rate or secondary endpoints, with aprinocarsen; however, the toxicity was not unduly increased, and aprinocarsen regimen was safely administered.
Similar content being viewed by others
References
Ferlay J, Bray P, Pisani P, Parkin DM: GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide (ed Version 1). IARC CancerBase No. 5, IARC Press Lyon, 2001
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, The Eastern Cooperative Oncology Group: Comparison of 4 chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med 346: 92–98, 2002
Dy GK, Adjei AA: Novel targets for lung cancer therapy: Part I. J Clin Oncol 20: 2881–2894, 2002
Davies AM, Gandara D, Lara P, Mack P, Lau D, Gumerlock P: Antisense Oligonucleotides in the Treatment of NSCLC. Clin Lung Cancer 4: S68-S73, 2003
Tamm I, Dorken B, Hartmann G: Antisense therapy in oncology: New hope for an old idea? Lancet 358: 489–497, 2001
Crooke ST: Basic Principles of Antisense Technology; In: Crooke ST (ed) Antisense Drug Technology: Principles, Strategies, and Applications. Marcel Dekker, New York, 2001, pp. 1–28
Basu A: The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther 59: 257–280, 1993
Dean NM, McKay R, Condon TP, Bennett CF: Inhibition of protein kinase C-alpha expression in human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J Biol Chem 269: 16416–16424, 1994
Lahn M, Sundell K, Moore S: Targeting protein kinase C-alpha (PKC-alpha) in cancer with the phosphorothioate antisense oligonucleotide aprinocarsen. Ann N Y Acad Sci 1002: 263–270, 2003
Yuen AR, Halsey J, Fisher GA, Advani R, Moore M, Saleh M, Ritch P, Harker G, Ahmed F, Jones CM, Polikoff J, Keiser W, Kwoh TJ, Holmlund JT, Dorr A, Sikic BI: Phase I/II trial of ISIS 3521, an antisense inhibitor of PKC-alpha, with carboplatin and paclitaxel in non-small cell lung cancer. (Abstract) Proc Am Soc Clin Oncol 20: 309a, 2001
Sikic BI, Yuen AR, Advani R, Halsey J, Fisher GA, Holmlund J, Dorr A: Antisense oligonucleotide therapy targeted to protein kinase C-alpha (ISIS 3521/CGP 64128A) by 21-day infusion: Results of the phase I trail and activity in ovarian carcinoma. (Abstract) Proc Am Soc Clin Oncol 17: 429a, 1998
Nemunaitis J, Holmlund JT, Kraynak M, Richards D, Bruce J, Ognoskie N, Kwoh TJ, Geary R, Dorr A, Von Hoff D, Eckhardt SG: Phase I evaluation of ISIS 3521 (ISI 641A), an antisense oligodeoxynucleotide to protein kinase C-α, in patients with advanced cancer. J Clin Oncol 17: 3586–3595, 1999
Sandler A. State-of-the-art treatment for advanced non-small cell lung cancer. Oncology 17: 15–22, 2003
Ritch PS, Belt R, George S, Valdivieso M, Figueroa J, McCachren S, Modiano M, Miller GL, Leonardo J, Dorr A, Oliver JW, Holmlund J, Otterson GA, Villanola-Calero MA: Phase I/II trial of ISIS 3521/LY9000003, an antisense inhibitor of PKC-alpha with cisplatin and gemcitabine in advanced non-small cell lung cancer (NSCLC). (Abstract) Proc Am Soc Clin Oncol 21: 309a, 2002
Lynch TJ, Raju R, Lind M, Riviere A, Gatzemeier U, Dorr A, Holmlund J, Yuen A, Sikic BI: Randomized phase III trial of chemotherapy and antisense, oligonucleotide LY9000003 (ISIS 3521) in patients with advanced NSCLC: Initial report. (Abstract) Proc Am Soc Clin Oncol 22: 623, 2003
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–16, 2000
Gatzemeier U, Groth G, Butts C, van Zandwijk N, Shepherd F, Ardizzoni A, Barton C, Ghahramani P, Hiwasa T: Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small cell lung cancer. Ann Oncol 15: 19–27, 2004
Andre F, Le Chevalier T, Soria JC: Her2-neu a target in lung cancer? Ann Oncol 15: 3–4, 2004
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA: Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. Morbidity & Mortality Weekly Report Recommendations & Reports 51: 1–29, 2002
World Health Organization: Guidelines on Prevention and Control of Hospital-Acquired Infections. SEA-HLM-343. New Delhi, India, World Health Organization, 2002
Anderson N, Lokich J, Bern M, Wallach S, Moore C, Williams D: A phase I clinical trial of combined fluoropyrimidines with leucovorin in a 14-day infusion. Demonstration of biochemical modulation. Cancer 63: 233–237, 1989
Lokich J, Bern M, Anderson N, Wallach S, Moore C, Beauchamp K, Williams D: Cyclophosphamide, methotrexate, and 5-fluorouracil in a three-drug admixture. Phase I trial of 14-day continuous ambulatory infusion. Cancer 63: 822–824, 1989
Peterson P, Iturria N, Sundell K, Lahn M: Meta-analysis of Infection During Continuous Infusion Chemotherapy With Minimal Infusate Changes. (Abstract) Lung Cancer 41 (Suppl): 140S, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vansteenkiste, J., Canon, JL., Riska, H. et al. Randomized phase II evaluation of aprinocarsen in combination with gemcitabine and cisplatin for patients with advanced/metastatic non-small cell lung cancer. Invest New Drugs 23, 263–269 (2005). https://doi.org/10.1007/s10637-005-6736-x
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-6736-x