Summary
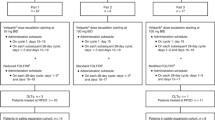
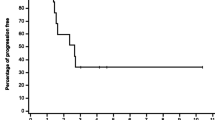
Background. Flavopiridol is a cyclin dependent kinase inhibitor. Preclinical models suggest a sequence dependent synergy between flavopiridol and taxanes. The primary objective of this study was to determine the maximum tolerated dose (MTD) of flavopiridol and docetaxel and the influence of flavopiridol on the pharmacokinetics of docetaxel. Methods: The major eligibility criteria included: a diagnosis of non-hematologic cancer with no conventional effective therapy, normal organ function, and ECOG performance status of 0-2. Patients were treated with docetaxel followed 24 h later by flavopiridol given via continuous intravenous infusion over a 24-h period. The starting doses of docetaxel and flavopiridol were 60 and 60 mg/m2, respectively. Cycles were repeated every 21 days. All patients received diarrhea prophylaxis consisting of bismuth subsalicylate. Results: Ten patients (M:F 4:6; median age 56 years) were treated. The median number of cycles per patient was 2 (range 1–6). Two of the three patients on dose level 1 developed dose-limiting toxicities consisting of neutropenia and fever. Seven patients were subsequently enrolled on dose level −1 (docetaxel 60 mg/m2, flavopiridol 50 mg/m2). One episode of grade 3 diarrhea was reported at dose level −1. Conclusions: Neutropenia complicated by infection was the major dose-limiting toxicity. The recommended doses of flavopiridol and docetaxel for phase II trials are 50 and 60 mg/m2 every three weeks, respectively.
Similar content being viewed by others
References
Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 100(1):57–0, 2000
Morgan DO: Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–91, 1997
Shah MA, Schwartz GK: Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin Cancer Res 7(8):2168–181, 2001
Senderowicz AM, Sausville EA: Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst 92(5):376–87, 2000
Sedlacek HH: Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol 38(2):139–70, 2001
Carlson B, Lahusen T, Singh S, Loaiza-Perez A, Worland PJ, Pestell R, Albanese C, Sausville EA, Senderowicz: Down-regulation of cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced by flavopiridol. Cancer Res 59(18):4634–641, 1999
Li Y, Bhuiyan M, Alhasan S, Senderowicz AM, Sarkar FH: Induction of apoptosis and inhibition of c-erbB-2 in breast cancer cells by flavopiridol. Clin Cancer Res 6(1):223–29, 2000
Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ: Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res 56(13):2973–978, 1996
Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK: Flavopiridol enhances the effects of docetaxel in vitro and in vivo in human gastric cancer cells. Mol Cancer Ther 2(6):549–55, 2003
Motwani M, Delohery TM, Schwartz GK: Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res 5(7):1876–883, 1999
Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, Hill K, Steinberg SM, Figg WD, Tompkins A, Arbuck SG, Sausville EA: Phase I trial of continuous infusion flavopiridol, a novel cyclin- dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol 16(9):2986–999, 1998
Thomas JP, Tutsch KD, Cleary JF, Bailey HH, Arzoomanian R, Alberti D, Simon K, Feierabend C, Binger K, Marnocha R, Dresen A, Wilding G : Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol 50(6):465–72, 2002
Schwartz GK, O’Reilly E, Ilson D, Saltz L, Sharma S, Tong W, Maslak P, Stoltz M, Eden L, Perkins P, Endres S, Barazzoul J, Spriggs D, Kelsen D: Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Oncol 20(8):2157–170, 2002
Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH: Taxanes: A new class of antitumor agents. Cancer Invest 13(4):381–04, 1995
Grant DS, Williams TL, Zahaczewsky M, Dicker AP: Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). Int J Cancer 104(1):121–29, 2003
Crown J: Docetaxel: Overview of an active drug for breast cancer. Oncologist 6(Suppl 3):1–, 2001
Leahy M, Howell A: Docetaxel. Br J Hosp Med 57(4):141–44, 1997
Lee SH, Yoo SD, Lee KH: Rapid and sensitive determination of paclitaxel in mouse plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 724(2):357–63, 1999
Schwartz GK, Ilson D, Saltz L, O’Reilly E, Tong W, Maslak P, Werner J, Perkins P, Stoltz M, Kelsen D: Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J Clin Oncol 19(7):1985–992, 2001
Stadler WM, Vogelzang NJ, Amato R, Sosman J, Taber D, Liebowitz D, Vokes EE: Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: A University of Chicago Phase II Consortium study. J Clin Oncol 18(2):371–75, 2000
Shapiro GI, Supko JG, Patterson A, A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clin Cancer Res 7(6):1590–599, 2001
Clarke SJ, Rivory LP: Clinical pharmackokinetics of docetaxel. Clin Pharmacokinetics 36(2):99-114, 1999
Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M: Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res 53(5):1037–042, 1993
Rosing H, Lustig V, van Warmerdam LJ, Huizing MT, ten Bokkel Huinink WW, Schellens JH, Rodenhuis S, Bult A, Beijnene JH: Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer Chemother Pharmacol 45(3):213–18, 2000
Bruno R, Riva A, Hille D, Lebecq A, Thomas L: Pharmacokinetic and pharmacodynamic properties of docetaxel: Results of phase I and phase II trials. Am J Health Syst Pharm 54(24 Suppl 2):S16–S19, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Rayes, B.F., Gadgeel, S., Parchment, R. et al. A phase I study of flavopiridol and docetaxel. Invest New Drugs 24, 305–310 (2006). https://doi.org/10.1007/s10637-005-4343-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-4343-5