Abstract
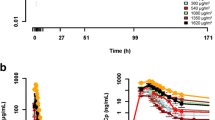
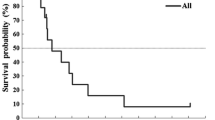
Purpose: To determine the anti-tumor activity DX-8951f when administered as a 30-minute infusion daily for 5 days every 3 weeks to patients with previously untreated metastatic gastric cancer, and to evaluate toxicities and pharmacokinetics (PK) of DX-8951f in this patient population. Patients and methods: Forty-one patients were enrolled. All had previously untreated metastatic gastric cancer. DX-8951f was administered until disease progression or unacceptable toxicity. Responses were assessed after every 2 courses using RECIST criteria. Results: Thirty-nine patients were evaluable. Two patients achieved a partial response (PR) and 18 achieved stable disease (SD), including five patients with unconfirmed PR. A total of 141 courses of therapy were delivered (median 3, range 1–10). The most common drug-related toxicity was neutropenia. Non-hematologic toxicities were mostly mild to moderate; the most common were nausea, vomiting and anorexia. Plasma concentrations of DX-8951 (the anhydrous form of DX-8951f) were well described using a linear 2-compartment PK model. All concentrations and dose events were simultaneously modeled and explained by the population PK model. There was no evidence of non-linearity in the elimination PK, auto-inhibition or induction of DX-8951 clearance over the five days of administration. Conclusions: DX-8951f had modest activity against metastatic gastric cancer and its PK was dose-proportional. The toxicity profile was predictable and manageable. Further development of this agent is warranted.
Similar content being viewed by others
References
American Cancer Society: Cancer Facts & Figures 2004. http://www.cancer.org
Agboola O: Adjuvant treatment in gastric cancer. Cancer Treat Rev 20: 217–240, 1994
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Yaguchi T: Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34: 1715–1720, 1998
Fischer DS, Knobf MT, Durivage HJ: The Cancer Chemotherapy Handbook, 4th ed. Mosby, St. Louis, 1993:302
Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, Sakata Y, Hyodo I: Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 17: 319–323, 1999
Saltz LB, Schwartz GK, Ilson DH, Quan V, Kelsen DP: A phase II study of topotecan administered five times daily in patients with advanced gastric cancer. Am J Clin Oncol 20: 621–625, 1997
Bendetti JK, Burris HA, Balcerzak SP, Macdonald JS: Phase II trial of topotecan in advanced gastric cancer: A Southwest Oncology Group study. Invest New Drugs 15: 261–264, 1997
Mitsui I, Kumazawa E, Hirota Y: A new water-soluble camptothecin derivative, DX-8951f, exhibits potent antitumor activity against human tumors in vitro and in vivo. Jpn J Cancer Res 86: 776–782, 1995
Kumazawa E, Jimbo T, Ochi Y, Tohgo A: Potent and broad antitumor effects of DX-8951f, a water-soluble camptothecin derivative, against various human tumors xenografted in nude mice. Cancer Chemother Pharmacol 42: 210–220, 1998
Takiguchi S, Kumazawa E, Shimazoe T: Antitumor effect of DX-8951, a novel camptothecin analog, on human pancreatic tumor cells and their CPT-11-resistant variants cultured in vitro and xenografted into nude mice [published erratum appears in Jpn J Cancer Res 88: 919, 1997]. Jpn J Cancer Res 88: 760–769, 1997
Daiichi Pharmaceutical Corporation: DX-8951f for injection: Investigators Brochure 9th ed. Fort Lee, NJ, September 22, 1998
Rowinsky EK, Johnson TR, Geyer CE Jr, Hammond LA, Eckhardt SG, Drengler R, Smetzer L, Coyle J, Rizzo J, Schwartz G, Tolcher A, Von Hoff DD, De Jager RL: DX-8951f, a hexacyclic camptothecin analog, on a daily-times-five schedule: A phase I and pharmacokinetic study in patients with advanced solid malignancies. J Clin Oncol 18: 3151–3163, 2000
De Jager R, Cheverton P, Tamanoi K, Coyle J, Ducharme M, Sakamoto N, Satomi M, Suzuki M: DX-8951f Investigators: DX-8951f: Summary of phase I clinical trials. Ann N Y Acad Sci 922: 261–273, 2000
National Cancer Institute (NCI) Common Toxicity Criteria (CTC) Version 2.0; January 30, 1998
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92: 205–216, 2000
Simon R: Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials 10: 1–10, 1989
Oguma T, Ohshima Y, Nakaoka M: Sensitive high-performance liquid chromatographic method for the determination of the lactone form and the lactone plus hydroxy-acid forms of the new camptothecin derivative DX-8951 in human plasma using fluorescence detection. J Chromatogr B Biomed Sci Appl 740: 237–245, 2000
Oguma T, Konno T, Inaba A, Nakaoka M: Validation study of assay method for DX-8951 and its metabolite in human plasma and urine by high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Biomed Chromatogr 15: 108–115, 2001
Collins DG, Forrest A: IT2S User’s Guide. State University of New York at Buffalo, Buffalo, 1995
Slichenmyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH: The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst 85: 271–291, 1993
Eng WK, Faucette L, Johnson RK, Sternglanz R: Evidence that DNA topoisomerase I is necessary for the cytotoxic effects of camptothecin. Mol Pharmacol 34: 755–760, 1988
Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y: Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res 49: 1465–1469, 1989
Kumazawa E, Tohgo A: Antitumor activity of DX-8951f: A new camptothecin derivative. Exp Opin Invest Drugs 7: 625–632, 1998
Joto N, Ishii M, Minami M, Kuga H, Mitsui I, Tohgo A.: DX-8951f, a water-soluble camptothecin analog, exhibits potent antitumor activity against a human lung cancer cell line and its SN-38-resistant variant. Int J Cancer 72: 680–686, 1997
Lawrence RA, Izbicka E, De Jager RL, Tohgo A, Clark GM, Weitman SD, Rowinsky EK, Von Hoff DD: Comparison of DX-8951f and topotecan effects on tumor colony formation from freshly explanted adult and pediatric human tumor cells. Anticancer Drugs 10: 655–661, 1999
Davidson K, Izbicka E, Lawrence C, Cerna C, Gomez L, Clark GM, De Jager RL, Weitman S, Von Hoff DD: Anticancer activity of DX-8951f, a water soluble camptothecin against human tumor specimens taken directly from adult and pediatric patients. Proc Am Soc Clin Oncol 17: 758, 1998 (abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a contract from Daiichi Pharmaceutical Corporation, Montvale, New Jersey.
Rights and permissions
About this article
Cite this article
Ajani, J.A., Takimoto, C., Becerra, C.R. et al. A phase II clinical and pharmacokinetic study of intravenous exatecan mesylate (DX-8951f) in patients with untreated metastatic gastric cancer. Invest New Drugs 23, 479–484 (2005). https://doi.org/10.1007/s10637-005-2907-z
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-2907-z